您当前的位置:检测资讯 > 科研开发
嘉峪检测网 2021-07-10 21:38
2021年5月份,FDA发布了一则MR相关的指南终版,提供了FDA关于在MR环境下评估医疗器械安全性和兼容性的测试建议,以及医疗器械标签中磁共振成像(MRI)安全信息的推荐格式。此指南取代了2014版的MR指南"Establishing Safety and Compatibility of Passive Implants in the Magnetic Resonance (MR) Environment"。
与2014版指南相比,此指南最大的变化在于以下几点:
适用产品范围更广了:
之前仅适用于无源植入类器械,现在适用于所有预期进入MR环境的医疗
器械,包括所有植入医疗器械、固定在患者身上或由患者携带的医疗器
械(例如,外部胰岛素泵、脉搏血氧仪)、在临床护理期间合理预计会
进入MR环境的医疗设备,以及所有打算进入MR环境的医疗器械。
这意味着很多医疗器的械都要考虑这个指南的要求了,整个设计开发过
程中都要注意符合这个指南的要求。
MR相关labeling的要求更具体了:
要求所有产品labelling上的MR labeling都要清晰易懂,且能够在制造商
网站上和/或通过电话获取到。不能使用MR Compatible这个标识,因为
这个标识容易造成误解,且已经被废弃了。
针对这个指南,FDA在6.24举办了一场网络研讨会,大家可以在之前文章中找到相关通知:
【FDA会议通知】2021.06.24 Webinar - 医疗器械在MR环境下的安全所进行的测试和标记
此研讨会相关的信息分享给大家:
1、医疗器械与磁共振环境的相互作用,概述如下图:
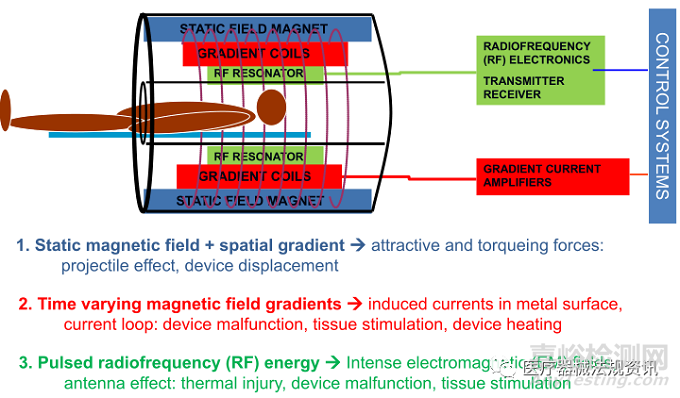
2、指南发展背景:
• Standardization of testing for passive implants
– American Society for Testing and Materials (ASTM) Test Standards
• Evolution of the Passive Implant Guidance
– “Establishing Safety and Compatibility of Passive Implants in the
Magnetic Resonance (MR) Environment,” dated December 11, 2014
• Safety testing for active implantable medical devices in the MR environment
– Covered currently by the ongoing ISO (International Organization for
Standardization)/TS (Technical Specification) 10974
• Identification of a need for a comprehensive guidance
• Draft guidance issued for comment August 2, 2019: “Testing and Labeling Medical Devices for Safety in the Magnetic Resonance (MR) Environment”
3、Relevant FDA Recognized Consensus Standards
• ASTM F2052: Standard Test Method for Measurement of Magnetically Induced Displacement Force on Medical Devices in the Magnetic Resonance Environment
• ASTM F2119: Standard Test Method for Evaluation of MR Image Artifacts from Passive Implants
• ASTM F2182: Standard Test Method for Measurement of Radio Frequency Induced Heating on or Near Passive Implants During Magnetic Resonance Imaging
• ASTM F2213: Standard Test Method for Measurement of Magnetically Induced Torque on Medical Devices in the Magnetic Resonance Environment
• ASTM F2503: Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment
• IEC 60601-2-33: Particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosis
• ISO/TS 10974: Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device
4、重要术语:
•磁共振(MR)环境(Magnetic Resonance (MR) environment)
–“磁共振磁体周围空间的三维体积,包含法拉第屏蔽体积和0.50 mT磁场轮廓(5高斯(G)线)。这里指的是医疗器械可能因暴露于磁共振设备和附件产生的电磁场而造成危险的区域。
•磁共振(MR)系统(Magnetic Resonance (MR) System)
MR设备的集合,附件,包括显示、控制、能源供应和受控访问区域(如提供)”的装置
•MR安全(MR Safe)
–“暴露在任何MR环境中不会造成已知危害的医疗设备。MR-Safe医疗器械由不导电、非金属和非磁性的材料组成。
•MR条件性(MR Conditional)
–“在规定条件下,包括静态磁场、时变梯度磁场和射频场条件下,在MR环境中证明安全的医疗器械。”
•MR不安全(MR Unsafe)
–“对MR环境中的患者、医务人员或其他人员造成不可接受风险的医疗设备”
5、MR环境中医疗器械需解决的危害
• Magnetically induced displacement force (ASTM F2052)
• Magnetically induced torque (ASTM 2213)
• Heating (ASTM F2182, ISO/TS 10974)
• Gradient induced vibration (ISO/TS 10974)
• Gradient induced electrical potential (unintended tissue stimulation) (ISO/TS 10974)
• Rectification of radiofrequency pulses (unintended tissue stimulation) (ISO/TS 10974)
• Medical device malfunction (ISO/TS 10974)
• Extent of image artifact (ASTM F2119)
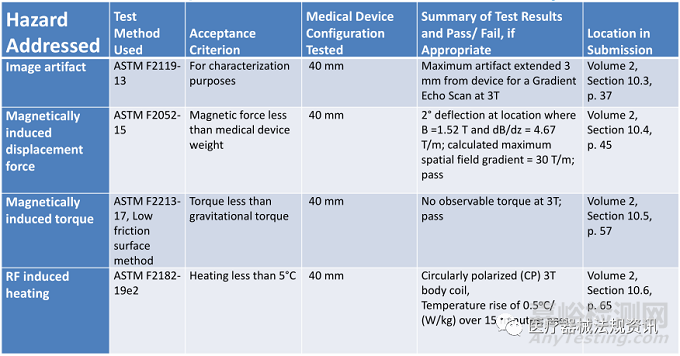
6、Reporting Results的要求
• Test report summaries, and if applicable, complete test reports should:
– List the hazard addressed by the test
– List the test equipment used
– Include results and all report elements as defined in the consensus standard.
• For the computational modeling report, follow the FDA Guidance: Reporting of Computational Modeling Studies in Medical Device Submissions .
• When ASTM F2182 is used, scale the values for °C/(V/m) or in °C/(W/kg) to a temperature increase (in °C) for the exposure conditions specified in the MR Conditional labeling.
• Provide a written narrative or a tabulated summary, including a narrative discussion of the results/conclusions
• See also the FDA guidance: Recommended Content and Format of Non-Clinical Bench Performance Testing Information in Premarket Submissions
Example of a Tabular Test Summary
7、MRI Safety Labeling的要求
The labeling should include:
• Sufficient information for a health care professional to determine whether a device can safely enter the MR environment
• A separate section of your labeling entitled “MRI Safety Information.”
• Based on assessments, MR Safe, MR Unsafe, or MR Conditional
• The appropriate symbol from ASTM F2503 and/or the corresponding term in the labeling
• A patient medical device card with MRI safety information
The MRI safety information should be readily accessible on the manufacturer’s website and/or by telephone.

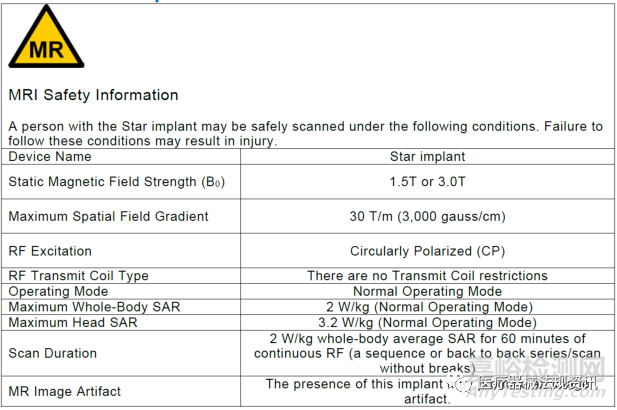
对于MR Conditional的设备,还有较多labeling的要求,大家可以细读指南要求了解更详细的要求。
Example MR Conditional labeling for a passive medical device
还有一类labeling,为Safety in MRI Not Evaluated的labeling,要求如下:
The labeling should include the following information:
“The <insert medical device name> has not been evaluated for safety in the MR environment. It has not been tested for heating or unwanted movement in the MR environment. The safety of <insert medical device name> in the MR environment is unknown. Performing an MR exam on a person who has this medical device may result in injury or device malfunction.”
Safety in MRI Not Evaluated的labeling的适用范围如下:
适用于预期在MR环境中使用的数量非常有限的医疗设备,例如某些无源植入物:
–以前没有提供任何关于MRI安全性的信息
–没有因暴露于MR环境而导致的已知不良事件
不适合标为Safety in MRI Not Evaluated的器械:
–通常被标记为MR Conditional或MR Unsafe
–新型的器械
–含有铁磁性材料
–有源类医疗器械
–部分植入的器械

来源:医疗器械法规资讯


