您当前的位置:检测资讯 > 科研开发
嘉峪检测网 2022-07-28 21:53
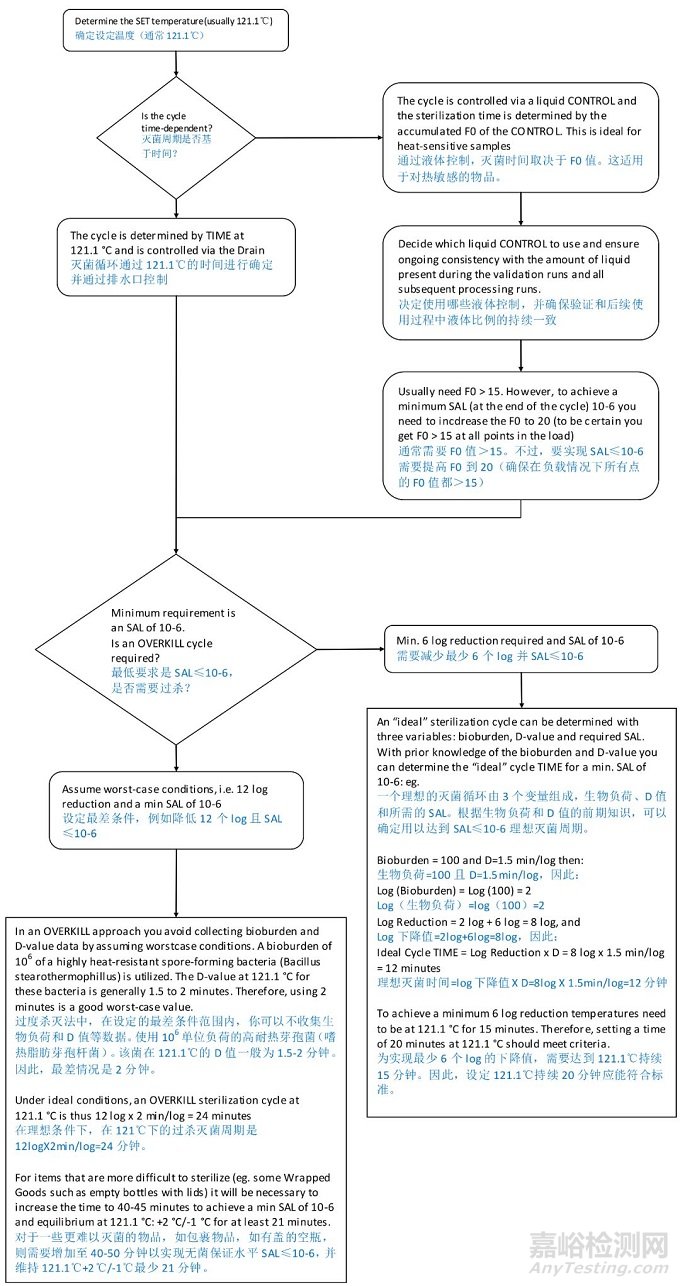
Consideration 1 Choosing the right sterilisation cycle to implement
注意事项 1:选择正确的灭菌周期
There are three basic types of sterilisation cycles. Choose the right one according to the type of goods to be sterilised:
有三种基本类型的灭菌周期。根据待灭菌物质的类型进行选择。
Hard Goods (Vacuum)
坚硬装载(真空)
Suitable for items that are easy to sterilise, because air removal and steam penetration are highly effective on these items. e.g., open glassware and large diameter piping
适用于那些易于灭菌的物质,因为空气清除和蒸汽穿透对这些物质是高度有效的。例如:敞开的玻璃器皿和大口径的管道
A typical hard goods cycle may draw one vacuum prior to introducing steam to reach the desired sterilisation temperature.
典型的坚硬物品灭菌循环在通入蒸汽达到设定灭菌温度前需要达到一个真空度。
Wrapped Goods (Vacuum)
包裹物品(真空)
Utilized for items that are difficult to sterilise, because air removal and steam penetration are harder to achieve on these items than on hard goods.
用于灭菌困难的物品,因为和坚硬品比起来空气清除和蒸汽穿透更难实现。
e.g., empty bottles (glass or plastic) with lids, gowns, long hoses/tubes, vent filters, portable vessels with small inlet/outlet ports
例如,有盖子的空瓶(玻璃或塑料),洁净服,长软管/管子,抽气过滤器,进/出口小的便携式容器。
A typical wrapped goods cycle may draw three or more vacuums prior to reaching sterilisation. A post-sterilisation vacuum draws the steam from the load items.
一个典型的包裹品灭菌循环在灭菌前至少进行 3 次抽真空。灭菌后通过真空将负载中的蒸汽抽走。
Liquids (Non-vacuum)
液体(非真空)
Items that contain liquids generally cannot have a deep vacuum pulled or the liquid will be drawn out of them. Autoclave cycles for liquids generally heat up and cool down without a vacuum. Steam, introduced into the top of the chamber, displaces the air. The air is pushed to the bottom of the chamber and is removed.
一般含有液体的物品不能有大的真空度,否则液体会溢出。针对液体来说高压灭菌锅循环是在没有真空度的情况下加热和冷却。蒸汽进入腔室顶部置换空气,空气推入腔室的底部并排出。
The steps involved in choosing the right sterilisation cycle.
选择正确灭菌循环的步骤:
Consideration 2 Which load configurations to use?
注意事项 2:使用哪一种负载配置
A variable to consider is whether to use fixed load or variable load configurations. There’s a trade-off here between validation effort and operational flexibility – do you want to validate a wide range of load configurations to increase Production’s flexibility in loading the autoclave? Here are some typical load configurations to consider:
需要考虑的一个因素是选择固定装载还是可变的装载。在验证和操作灵活性之间需要一个权衡- 你是否验证最大最小装载以增加生产的灵活性?下面是需要考虑的一些典型装载模式:
A fixed load/fixed position configuration means that any load to be sterilised will be placed inside the chamber in exactly the same way for every processing run. A diagram of the load configuration should appear in the Standard Operating Procedure (SOP) so that operators can reproduce the load for every processing run. This situation requires the fewest validation runs (3), but offers no flexibility in load configurations.
固定装载/固定位置模式 意味着, 任何待灭菌装载将以完全相同的方式放置在室内。在标准作业流程(SOP)中应有负载配置的图表,因此操作者可以在脑海中再现每一个运行过程的负载。这种情况要求最少的验证运行(3),但负载配置没有灵活性。
A fixed load/variable position configuration means that the location of the load items in the autoclave can vary. Only a list of the items that can be in a load is required for the SOP. The validation runs must demonstrate positional equivalence by rotating the items from location to location during the test runs. If positional equivalence is proven after three validation runs, then you can stop. A fixed load/variable position configuration gives operators flexibility in loading the autoclave. This saves time when loading large loads of numerous items of different types.
固定负载/可变位置模式 指负载在高压灭菌器内的位置是可变的。在 SOP 中只需一个负载列表。在测试运行过程中,验证运行应通过轮换负荷物品逐个位置论证不同位置的等效性。如果经过 3 个run的验证证明了位置的等效性那就可以停止。固定负载/可变位置的模式给操作者操作高压灭菌器时带来灵活性。针对包含不同物品的大负载来说,这样可以节省时间。
A variable load configuration means that different combinations of items and/or numbers of any item(s) can be placed into the chamber. The validation runs must demonstrate that the cycle is adequate for both a maximum and minimum load configuration. The minimum load tests are done with only one item in the autoclave, that item being the load item demonstrated as being the most difficult to sterilise .
可变负载配置 指腔室内物品种类和/或数量不固定。验证运行时必须表明此循环周期可以满足最大和最小两个负载模式。最小负载测试高压灭菌器腔室一个物品,必须证明其是最难灭菌的一个物品。
Consideration 3 Choosing the right Control for liquid cycles
注意事项 3:选择合适的液体循环监控装置
The choice of the Control used when sterilising liquids determines whether the load you are sterilising will pass all the acceptance criteria.
液体灭菌时所选的监控决定了待灭菌的负载能否通过接收标准。
More than one liquid Control may be needed to validate all the different types of bottles and liquids requiring sterilisation. Consider the following when choosing the Control for liquid cycles:
需要不止一个液体监控装置以证明不同类型的瓶子和液体能满足灭菌要求。选择液体循环监控装置时需考虑以下因素:
The size of the bottle and its fill volume – the larger the bottle and the greater its volume, the harder it is to sterilise.
瓶子的大小和填充量-瓶子越大其体积越大,就越难灭菌。
The thickness of the glass –thicker glass is more difficult to sterilise than thinner glass.
玻璃的厚度,厚玻璃比薄玻璃难灭菌
The viscosity of the liquid – the greater the viscosity, the slower the heat-up time, and the harder it is to sterilise.
液体的粘度 – 粘度越大传热越慢,就越难灭菌。
The liquid Control will ideally be the one that is the most difficult to sterilise (worst-case) and will be located at the coldest spot in the chamber (lower level near the front door or directly above the drain).
理想情况下,液体监控装置所在应是最难灭菌的(最坏情况),放置在腔室内最冷点(靠近前门或在排水口正上方)。
Don’t be tempted to use a Control that is dramatically different from the composition of the load. If the liquid Control takes too long to reach the sterilisation temperature, then the protein composition of the media in the rest of the load (which may have exceeded the desired temperature by the time the Control reaches the sterilisation temperature) may be denatured.
不要试图使用与待灭菌装载完全不通过的液体监控装置,如果液体控制需要很长时间来达到灭菌温度,则负载其他位置中的蛋白质可能会变性(在监控装置到达灭菌温度的过程中,可能已经超出所要求的温度)。
If in doubt, perform preliminary studies using different liquid Controls to obtain information on the load's heat-up times and F0-values.
不要对不同组分的负载尝试控制,如果液体控制需要很长时间还达不到灭菌温度,则负载介质中的蛋白质将会变性(这可能已经超出了控制的时候达到杀菌所需的温度)。
The number of validation runs required for different types of liquids and bottles can be reduced by grouping liquids with similar viscosities, bottle sizes and fill volumes. Each liquid Control will have a unique maximum and minimum load configuration associated with it.
可以根据相似粘度,瓶子大小和填充量大小进行分组,减少不同类型的液体和瓶子所需验证测试的次数。每个液体控制将有一个与之相关的最大和最小负载配置。
Use procedural controls to ensure that the choice of liquid, bottle size and fill volume used for each Control, and its location in the chamber, are maintained during the validation runs and subsequent processing runs.
使用程序化的控制,以确保每个液体控制所使用的液体、瓶子体积、填充量,及其在腔室内的位置,在验证期间和后续操作过程中保持一致。
Consideration 4 Determining which load items are the most difficult to sterilise and which location(s) within the items represents the worst-case conditions
注意事项 4:确定哪些装载物品是最难灭菌的和哪些位置最能代表灭菌的最差情况
With a large load containing a wide variety of different types of items, the number of possible test locations within items seems to approach infinity. It also can be difficult to get the thermocouple and indicators (BI & CI) into the item without affecting the item’s ability to be sterilised and/or ruining the item (a concern with expensive items).
由于负载包含很多不同类型物品,需要检测的位置似乎很多。在不影响物品灭菌能力或破坏物品(关注贵重物品)的情况下,将热电偶和指示剂(BI 与 CI)放入物品中也是非常困难的。
We must evaluate each item on a case-by-case basis and determine how to best challenge the item. Often the item must be sealed somehow to return it to a state that represents equivalency with respect to steam penetration.
我们必须逐个逐个评估每个物品,确定最佳挑战方式。通常,物品必须以某种方式密封,以确保其蒸汽渗透状态不变。
Some examples:
举例:
Q. What is the most difficult point to sterilise in a hose of uniform diameter?
问:一个直径均一的软管内哪一点最难灭菌?
A. In the centre of the length of hose.
答:在软管长度的中心。
Q. How do you get a 3 m length of thermocouple into the middle of a 20 m hose?
问:如何将一个 3 米长的热电偶插入 20 米的软管中间?
A. By cutting a slot in the middle of the hose and inserting the thermocouple through the slot, making sure to seal the slot with silicon. If you don’t seal it, you will not be challenging the hose properly. Alternatively, get two 10 m lengths of hose (if available) and join them with a connector, after inserting the thermocouple through the connector. Using this method doesn’t ruin a 20 m length of hose.
答:在软管中间刻一插槽,热电偶沿着插槽插入软管,确保用硅胶将插槽密封,如果你没有密封,则不能挑战软管。或者,将两个 10 米的软管(如有)插入热电偶后用连接器将其连接,用这种方法则不用破坏 20 米长的管子。
Q. What is the worst-case location within a bottle, flask or cylinder?
问:瓶子、锥形瓶和柱形瓶那个位置最难灭菌?
A. In the centre near the bottom (but not touching the floor).
答:在靠近底部的中间(但不接触底面)。
Q. How do you hold a thermocouple in position inside a sealed bottle?
问:如何保持热电偶在一个密封瓶子里的位置?
A. Choose a piece of Silastic tubing with an internal diameter (ID) that is narrow enough to hold the thermocouple probe without letting it slip through. Drill a hole in the bottle's lid the same size as the outer diameter (OD) of the tubing. Push the tubing through the hole, into the bottle. Now push the probe into the tubing. Slide the probe through the tubing until it reaches the desired position in the bottle. Make sure it is not touching the wall of the bottle. For bottles with rubber stoppers, make a small hole in the centre of the stopper, sufficient to push the thermocouple through.
答:选择一个内径与热电偶探头相近的硅橡胶管件套住它以确保不会滑落。在瓶盖上钻一个和管子外径一样大的孔。通过孔将管子插入瓶中。将探头推入管子中,推动探头直至到达瓶子中所需位置。确保它不会触碰瓶壁。对于橡胶瓶塞的瓶子,在筛子中间钻一个小孔,足以使热电偶通过。
Consideration 5 Wired temperature thermocouples are cumbersome and don’t always give accurate data
注意事项 5:有线热电偶较复杂,且并非总是提供准确的数据
The list below highlights some considerations when using wired thermocouples:
下面重点介绍一些使用热电偶传感器时的注意事项:
Some loss of steam (leakage) will occur when the wire's outer plastic protector has been cut and air or steam can pass through it. This may result in a failed leak test.
当导线的外层塑料保护膜被切割并且有空气或蒸汽通过,就会发生一些蒸汽损耗(泄漏),可能会导致泄漏测试失败。
Validator thermocouples inside the chamber will draw condensate and will need a slice/cut made in their outer protective layer, to ensure that any fluid is released. If condensate passes through the wires and into the electronics, the thermocouples will be destroyed.
在腔室内的热电偶会吸冷凝水,需要在腔室外部将热电偶保护层切开以将冷凝水释放。如冷凝水通过热电偶流到验证仪电子元件,热电偶将损坏。
The thermocouple may be difficult to place into the item without adversely affecting the item’s ability to be sterilised and/or ruining the item (a concern with expensive items).
热电偶可能难以在不影响物品灭菌和/或破坏物品 (贵重物品)的情况下,放入物品中。
Wires can get caught (and be damaged) under the autoclave's wheels when moving loads into and out of the chamber.
当进料或出料时热电偶可能被高压灭菌器的轮子卡住(并损坏)
It is difficult to place wires inside sealed bottles without (i) touching the inside wall, and (ii) compromising the bottle’s ability to be sterilised.
将热电偶装在密封瓶里并且不碰到内壁是很难做到的,并且降低瓶子的无菌程度。
You may be limited by the number of wires you can place through the autoclave's inlet.
你可能会受限于高温灭菌器进口的导线数量。
The resistance of the thermocouple in some locations in the chamber can change, leading to inaccurate and/or unreliable data even though the pre/post calibration verifications meet specifications.
即使前/后校准确认符合标准,某些位置热电偶电阻也会发生变化,从而导致数据不准确和/或不可靠。
Case Study
示例
This example looks at nine thermocouples placed into a loaded chamber. They were evenly spaced from one another at the top, middle and bottom levels and at the front, centre and rear of the chamber.
此示例包含装载腔室内的9个热电偶。他们均匀分布在顶部,中部和底部,以及前、中、后部。
The study used wired thermocouples in a loaded chamber for a 40-minute cycle at 121.1 °C. The chamber's maximum pressure of 2.16 bar (at any time) was equivalent to 122.7 °C. One probe (top front LHS) constantly reached temperatures between 123.3 °C and 123.5 °C. All other probes were within the required limit of 120.1 °C +2 °C/-1 °C at temperatures from 122.4 °C to 122.7 °C.
该研究使用有线热电偶用于测试 121.1℃ 40 分钟的负载腔体 。内室最大压力 2.16bar(在任何时间)相当于 122.7℃。一个探头(顶部前左)温度持续维持在123.3 °C - 123.5 °C之间。所有其他探头则在规定温度范围120.1℃ +2 ° C/-1 ° C内,在122.4 ° C 至 122.7 ° C 之间
The temperature differential started during heat-up and remained during the sterilisation and post vacuum cooling phases. The Equipment Engineer and the Manufacturer agreed that the temperature reading at this position was inaccurate and unreliable. The thermocouple reading was inconsistent with the steam pressure indication and the other thermocouple readings. Consequently, no useful data were collected at that point.
温度偏差开始于加热阶段,并在灭菌和真空冷却阶段仍然存在。该设备工程师和制造商一致认为,在这个点的温度读数是不准确和不可靠的。热电偶读数与蒸汽压力读数以及其他热电偶读数不一致。因此,在这一点没有任何有用的数据。
Consideration 6 Determining the acceptance criteria
注意事项 6:确定接受标准
An example:
一个例子:
You run your validation studies, only to realise that you cannot meet one of the acceptance criteria. But, was it really needed in the first place?
当您运行验证测试时,您只认识到能不能达到接受标准。但是,是不是真的需要摆在首位?
It’s important to understand the aim of the autoclave cycle and what its parameters are. For example, is it for sterilisation or decontamination? Is the load heat sensitive, or can it be subjected to an overkill cycle? Is it a porous load (hard/wrapped goods), or is it a liquid?
重要的是要了解灭菌柜的周期及其参数。例如,是用于灭菌还是降低负荷?负载是热敏感的吗?还是可以承受过度灭菌?是多孔装载(坚硬/包裹的物品)吗?,还是液体?
Most Validation departments have a Standard Operating Procedure (SOP) detailing the validation requirements for sterilisation processes. Included in that is a complete list of all the acceptance criteria.
大多数验证部门有标准操作程序(SOP)详细说明灭菌工艺验证的要求。列出所有接受标准的完整清单。
Each phase of the autoclave cycle is likely to have different acceptance criteria:
灭菌柜不同阶段的循环周期存在不同的遵循标准:
Phase I – Heat distribution (empty chamber)
第一阶段 - 热分布(空载)
Phase II – Heat distribution (loaded)
第二阶段 - 热分布(装载)
Phase III – Heat distribution (loaded) cold spot determination within
第三阶段 - 热分布(装载)内部冷点测定
Phase IV – Heat penetration
第四阶段 - 热穿透
There may also be different requirements for Phases III and IV if you are sterilising liquids (non-vacuum) vs. porous items (vacuum), e.g., F0 > 15 at the end of sterilisation (liquids only).
对于灭菌液体(非真空)和多孔装载(真空),第三阶段和第四阶段可能存在不同的要求,例如灭菌结束时 F0>15(只针对液体)。
Typical acceptance criteria are as follows:
典型的接受标准如下:
All porous cycles require min SAL 10-6 at the end of sterilisation. All porous items are subject to at least one post-vacuum cycle which removes steam from the chamber (Phases III & IV).
所有多孔装载程序要求结束时SAL≤10-6。所有多孔物品至少进行一次后真空循环以消除灭菌腔室内的蒸汽(阶段三和四)。
All liquid cycles require a min SAL 10-6 and a min F0 > 15 at the end of the cycle, because they do not use vacuum and are subject to natural cooling(Phases III & IV).
所有液体循环周期要求SAL≤ 10-6,并且F0 > 15,因为它们不使用真空,并自然冷却(阶段三和四)。
Throughout the sterilisation phase all temperatures are within a 3 °C range (Phases II, III & IV), e.g., 121.1 °C -1 °C/+2 °C.
在整个灭菌阶段所有的温度都在 3 ° C 的变化范围内(阶段二,三和四)例如,121.1 ° C -1℃/+2 ° C
Throughout the sterilisation phase all temperatures in the chamber are within 1.0 °C of the chamber's mean temperature (Phase II).
在整个灭菌阶段,灭菌柜各探头温度在平均温度±1.0 ℃范围内,(阶段二)。
The steam's temperature corresponds to its vapour pressure (Phases II, III & IV).4
蒸汽的温度与其蒸气压力相适应(阶段 II,III 及 IV)。
Timed measurements are to be controlled to an accuracy of ±1%.
时间计时控制在±1%的精度范围内(阶段 II,III 及 IV)。
Required pre-certification and post-certification of the data logger ensures that the temperature measurement system is accurate to within ±0.5 °C.
验证前和验证后温度测量系统的准确度在± 0.5℃的范围内。
The load is visually dry at the end of the cycle (porous cycles only).
灭菌结束时装载目测干燥(只针对多孔装载)。
All autoclaved Biological Indicators (BIs) are negative and the control is positive following incubation (Phase IV).
所有生物指示剂培养结果阴性,阳性对照阳性(阶段IV)。
Consideration 7 Adequately documenting the validation test runs
注意事项 7:充分记录验证测试
Documenting what was done during the validation test runs is all about knowing what needs to be documented and how to present it. This documentation must be clear, consistent between runs and transparent, and must conform to all GMP requirements. It must be complete and must include the following items:
记录验证测试期间进行的工作,就是了解需要记录的内容以及如何记录它。记录清晰、一致和一目了然,并且必须符合所有 GMP 要求。它必须是完整的,并且必须包括以下项目:
a diagram showing the location of all load items within the autoclave chamber
显示高压灭菌腔室内所有装载物品位置的图表
the precise location/number of each thermocouple, BI and CI within each item
各热电偶、BI、CI的数目和精确位置
the printout from the data recorder
数据记录器(热电偶)打印输出
the printout or chart from the autoclave
从灭菌器打印或输出图表
the time the sterilisation period began and finished (per the data recorder time)
灭菌周期起始和结束(根据数据记录器的时间)
the time difference between the autoclave controller and the validation temperature monitoring device
高压灭菌柜控制器和温度验证仪的时间差
the results of each BI and CI
各生物指示剂和化学指示剂的结果
Label each document with the equipment ID, load description, date, test run number and cycle start/end time.
每个记录分别标明设备 ID、负载说明、日期、测试编号和循环开始/结束时间。
If you fail to generate good documentation while conducting the validation test runs, you will not be able to analyse the data when putting together the report. Inadequate or poor quality data to support the validation process will not survive the scrutiny of an auditor.
当进行验证测试过程中,如果你没有良好记录,在报告时,你将无法进行数据分析。用不充分或不良的数据来支持验证过程将无法通过审计。
Tip:
提示
Be cautious about the acceptance criteria you employ to verify the accuracy of thermocouples. If the criterion is too tight (e.g., all thermocouples must meet the acceptance criteria), you may lose a lot of runs if one or two thermocouples cease functioning or are outside the temperature tolerance after the runs.
注意谨慎使用热电偶的精确度接受标准,如果标准过高(如所有的热电偶必须达到接受标准),如有一或两个热电偶停止工作或在测试后超出温度容许偏差,则可能会丢失大量运行。
Consideration 8 The frequency of thermocouple accuracy verification
注意事项 8:热电偶准确度确认的频率
If you are performing a large number of test runs (e.g., over the course of several weeks), you need to think about the points at which you will verify the thermocouples' accuracy. This could be done after every run and/or at the end of the entire testing period. If you wait until the end of the testing period, you run the risk that all of the runs are of no value due to their failure to meet the verification acceptance criteria. Verifying after every run, however, adds considerably to the length of time required to complete the testing. Performing the verification every three runs or every few days is a reasonable compromise.
如果你正在进行(如,在几个星期内)大量的验证测试,你需要考虑热电偶的准确度。可以在每次运行和/或在整个测试周期结束后进行。如果等到测试周期结束,则存在由于热电偶未满足接受验收标准而导致所有测试失效的风险。但是,在每次测试后做检查,则会大大增加完成测试所需的时间。每三次运行或每隔几天执行一次准确性检查是一种合理的折衷方案。
As noted in Consideration 6, the acceptance criterion you employ to verify the thermocouples' accuracy should allow at least one or two thermocouples to fail.
正如注意事项 6 指出,验证热电偶的准确度,应允许至少一个或两个热电偶失效。
Consideration 9 Having adequate time and access to the autoclave to complete the validation
注意事项 9:确保足够的时间和支配来完成验证
It’s easy to under estimate the length of time it takes to validate an autoclave, and how much access you need to it during the process.
人们很容易低估验证高温灭菌器所需的时间,以及在整个验证过程中你需要做些什么。
For example, it can take up to four hours to set up your run, i.e., prepare the load, place probes, BIs and CIs into the load, etc. If Production needs to use the autoclave and you need to remove your probes, BIs and CIs, then you need to start all over again, effectively losing a day. Work with the Production department when planning the Validation project to ensure that you have adequate access to the autoclave.
例如,可能需要长达四个小时进行准备,例如,准备装载,放置探头,将BI和 CI 放入负载中等。如果生产需要使用高压灭菌器,你需要将探头,BI 和 CI挪出来,然后从头再来,白白浪费一天。在规划验证项目时与生产部门合作,以确保你能有足够的支配。
Another approach is to combine Phase II and III (Heat Distribution) with Phase IV (Heat Penetration) studies to save time.
另一种方法是将阶段II、阶段III(热分布)与阶段IV(热渗透)结合在一起,以节省时间。
Combining these three phases could reduce the time it takes to complete the validation project; however, you need to consider the following when doing this:
这三个阶段结合一起做可以减少完成验证的时间,不过,这样做需要考虑以下几点:
1 You will need to place probes into the chamber and into load items at the same time. Can you fit all the probes through the autoclave's inlet? If not, then you need to either validate each phase separately, or reduce the number of probes.
1、需要同时将探头放入墙体内和负载物上。通过高压灭菌器验证口你能安装所有的探头吗?如果不能,那么你需要单独验证每个阶段,或降低探头的数量。
2 Combining these three phases greatly increases your preparation time. If you are working on a tight schedule (e.g., on a construction site where you need to evacuate at a certain time), you may not have time to complete the study. If this happens, then it may take more time to perform the work than if you had done each phase separately.
2、这三个阶段结合在一起做会大大增加你的准备时间。如果你的时间较紧(例如,在需要在特定时间撤离的建筑工地上),,你可能没有时间完成这项分析。如果发生这种情况,那么它需要完成的时间比单独做每个阶段的时间要长。
3 If you are under time pressure, there is a greater chance that you will miss something or make a mistake.
3、如果你的时间很紧,将很容易遗忘一些东西或者犯错误。
4 There are more data to consider. If you check only a few critical requirements before proceeding to the next study, you may miss something that did not meet an acceptance criterion. This may mean that all subsequent studies are at risk, because the data cannot be verified. For example, a probe may be falsely reading too high.
4、还有更多的数据需要考虑。进行下一个研究前,如果你只检查了几个关键点,您可能会疏忽一些不符合验证标准的内容,这可能意味着所有后续的研究处于风险之中,因为数据无法得到证实。例如,一个探头读数可能过高。
5 Allow enough time for the report to be completed.
5、准备充足的时间来完成报告。
If you are validating a new autoclave, then you need to allow enough time for:
如果你是对新的高压灭菌器进行验证,那么你需要足够的时间进行:
(一)writing a validation plan
(一)编写验证计划
(二)writing the commissioning and IQ protocols
(二)编写试机和 IQ方案
(三)preparing the OQ/PQ protocols
(三)准备 OQ / PQ方案
(四)performing OQ/PQ studies
(四)开展 OQ / PQ 的研究
(五)writing the OQ/PQ reports, preparing folders, etc.
(五)编写 OQ / PQ 的报告,准备文件等
If you are performing a large number of test runs (e.g., over the course of several weeks), then you need to ensure that enough time has been allocated to prepare the folders and write the reports. Allow one day to do a run and another day to analyse the data, i.e., two days per study. Also allow time to write protocols and reports, and to have them reviewed and approved by other people (if appropriate). If you are developing the validation cycles, then this will also take time.
如果你正在进行(例如,在几个星期内)大量运行测试,那么你需要确保有足够的时间准备文件和编写报告。确保测试一天,再用一天来分析数据,也就是说,每项测试2天。同样预留充足的时间编写方案和报告,并进行审核签批(如适用)。如果你正在开发验证程序,那么这也将需要时间。
Consideration 10 Have the right procedural controls in place to ensure ongoing consistency and correct operation
注意事项 10:制定正确的程序进行控制,以确保持续一致和正确操作
Congratulations, you have just finished validating a new autoclave for a number of different cycles and load configurations. Now, what controls need to be in place to ensure that the validated loads are used consistently?
恭喜你,你刚刚完成了一个包含不同周期和负载的新灭菌柜的验证。现在,需要那些控制,确保一致地使用经验证的装载?
A Standard Operating Procedure (SOP) for the new autoclave should be prepared. It must include clear guidelines for each of the validated cycles, including diagrams of the load configurations. Test the procedure's clarity by asking a typical operator to follow the instructions with a dummy load.
准备新高温灭菌器的标准操作程序(SOP)。对每个验证程序必须有明确的指引,包括负载图。应准备新高压灭菌器的标准操作程序 (SOP)。它必须包括每个经过验证的周期的明确准则,包括负载配置图。要求一个典型操作员按照SOP进行操作来检查SOP的清晰度。
Each operator who uses the autoclave should be trained and tested on the SOP.
对每个使用高压灭菌器操作者进行培训和 SOP 考试。
Logbooks should be in place for each cycle.
每个程序都应有记录。
Use a risk-based approach to determine the troubleshooting guidelines to include in the SOP. The manufacturer’s documentation and website may detail things that commonly go wrong.
采用基于风险评估的方法制定检修指南,包含在 SOP 里。制造商的文件和网站可能有常见故障的详述。
An ongoing requalification program for the autoclave and the loads is required. The frequency can be 6, 12 or 24 months.
对于高压灭菌器和装载来说,持续再确认程序是必需的,频率可为 6,12 或 24 个月。

来源:GMP办公室


