This is an example pharmaceutical development report illustrating how ANDA applicants can move toward implementation of Quality by Design (QbD). The purpose of the example is to illustrate the types of pharmaceutical development studies ANDA applicants may use as they implement QbD in their generic product development and to promote discussion on how OGD would use this information in review.
FDA官网中一个有关药物开发报告的实例,用以说明申请人如何实施质量源于设计(QbD)。 该实例的目的是说明ANDA申请人在其仿制药开发过程中实施QbD时,可使用的药物开发研究的类型,同时促进探讨OGD在审评中如何使用该信息。
2.3.6 Scale-Up from Lab to Pilot Scale and Commercial Scale
从实验室规模放大至中试规模和工业规模
Note to Reader: Currently, scale-up information is limited at the time of submission. The applicant should discuss product specific scale-up principles including their planned approach to scale-up the process. OGD will evaluate the applicant’s plan to determine its adequacy. However, if a substantial amendment needs to be submitted due to the inadequacy of the scale-up plan, it may significantly extend the review process. It is the firm’s discretion to submit scale-up data such as actual process verification information at the time of submission for a complex drug product which has a high risk of scale-up failure; however, in some cases it may be requested by OGD.
通常,递交时的放大信息是有限的。申请人应讨论产品具体放大原则包括它们计划 放大工艺的方法。OGD将评估申请人的计划以决定其适宜性。但是,如果需要提交实质性 修正由于放大计划不适宜,则可显著延长审查过程。对于公司可酌情递交放大数据如递交具 有放大失败高风险的复方药品时的实际工艺验证信息;但是,某些情况下,OGD可能要求 递交。
Process development was conducted on the lab scale (5.0 kg). This section describes the principles used to scale-up the process to the pilot scale (50.0 kg) in order to manufacture the exhibit batch. The same principles will be employed to scale-up the process to the commercial scale uponapproval. Table 50 summarizes the different process scales.
进行了实验室规模(5.0 kg)的工艺开发。本节描述了用于放大至中试规模(50.0 kg)的工艺原则 以便生产申报批。一旦批准,将使用相同原则用于放大至工业规模的工艺原则。表 50 总结 了半天的工艺规模。
2.3.6.1 Scale-Up of the Pre-Roller Compaction Blending and Lubrication Process
预碾压混合和润滑工艺的放大
The process development work for the pre-roller compaction blending and lubrication step was carried out in a 16 qt capacity twin shell V-blender. To scale-up, it was desirable to maintain geometric, dynamic and kinematic similarity by applying the following rules: 预碾压混合和润滑步骤的工艺开发工作在16 qt容量双筒V型混合机内进行。为达到放大,通 过使用如下规则来维持几何相似,动力相似和运动相似是可行的:
● Geometric similarity: keeping the ratio of all lengths constant (constant fill ratio) 几何相似:所有长度比保持恒定(填充比恒定)
● Dynamic similarity: maintaining constant forces (Froude number Fr) 动力相似:维持恒力(Froude数Fr)
Fr= rpm2R/g
rpm: revolutions per minute 每分钟转数 R:characteristic radius 特征半径 g:gravitational constant 引力常数
● Kinematic similarity: maintaining a consistent number of revolutions (rpm × minutes) 运动相似:维持转数稳定(rpm ×分钟)
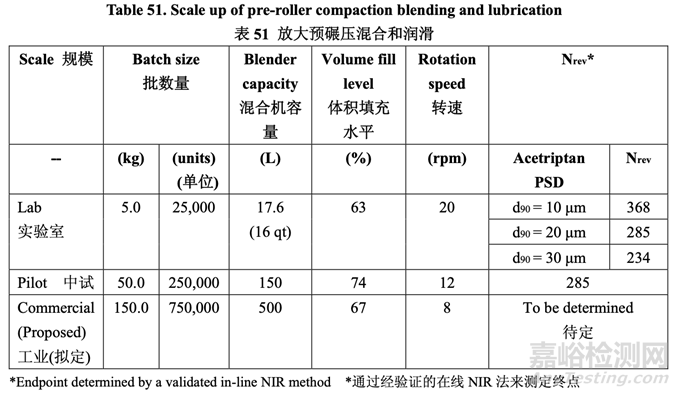
At the pilot scale, the fill level was 74%. This was slightly higher than the fill level at lab scale which was 63%. The rotation speed at both scales was fixed due to equipment constraints. Although the target blending endpoint could be estimated by maintaining similarity between the scales, the final endpoint was determined using the validated in-line NIR method (details provided in Section 3.2.P.5.3 Validation of Analytical Procedures). To assess homogeneity of the blend, a moving block % RSD was calculated for each moving block of ten consecutive spectra and plotted as a function of time. The blend was considered uniform once the % RSD was below 5% for ten consecutive measurements.
在中试规模,填充水平为74%。这稍微比实验室填充水平(63%)高。由于设备限制,固定了 两个规模的转速。虽然目标混合终点可通过维持两个规模间的相似性来估计,但使用经验证 的在线NIR法(具体见3.2.P .5.3节分析方法验证)来测定最终终点。为评估混合物的均匀性,计 算10个连续光谱中每个移动区的移动区%RSD并根据时间绘图。一旦10个连续测量值的 %RSD低于5%,可认为混合均匀。
The pre-roller compaction blending and lubrication process scale-up is summarized in Table 51.
表 51 总结了预碾压混合和润滑工艺的放大。
2.3.6.2 Scale-Up of the Roller Compaction and Integrated Milling Process
碾压和集成粉碎工艺的放大
For this drug product, the roller compaction process first needed to be scaled up from lab scale (using Alexanderwerk WP120 with 120 mm roll diameter and 25 mm roll width) to pilot scale (using Alexanderwerk WP120 with 120 mm roll diameter and 40 mm roll width) and then, ultimately, to commercial scale (using Alexanderwerk WP200 with 200 mm roll diameter and 75 mm roll width).
对于该制剂,碾压工艺首先需要从实验室规模(使用轧辊直径为120 mm和轧辊宽为25 mm的 Alexanderwerk WP120)放大中试规模(使用轧辊直径为120 mm和轧辊宽为40 mm的 Alexanderwerk WP120),并最终放大至工业规模(使用轧辊直径为200 mm和轧辊宽为75 mm 的Alexanderwerk WP 200)。
In a roller compaction process, there are several process parameters to consider when scaling up to a larger, wider roller. The strategy employed for each process parameter is discussed below. 碾压工艺中,当放大至较大,较宽轧辊时,需考虑几个工艺参数。每个工艺参数使用的策略 讨论如下。
Roller Gap 轧辊间隙
The scale-up strategy for the roller gap was to maintain the ratio between the roller gap (S) and the roller diameter (D) for different size roller compactors. The scale-up factor for the roller gap was calculated according to the following equation: 轧辊间隙的放大策略是维持不同大小压实机的轧辊间隙(S)和轧辊直径(D)的比。按照如下方 程计算轧辊间隙的放大系数:
S1/D1= S2/D2
Roll Force or Roll Pressure 轧辊力或轧辊压力
Based on the process development work, ribbon density was an intermediate critical quality attribute for this process step and strongly affected the downstream compression force required to meet the target tablet hardness. A commonly used strategy to scale-up roller compaction is to control the ribbon density by maintaining the roller peak pressure (Pmax) as described by Johanson’s model.
基于工艺开发工作,带状物密度是该工艺步骤的中间体关键质量属性,强烈地影响需符合片 剂目标硬度的下游压缩力。放大碾压的常用策略是通过维持轧辊峰压力(Pmax)来控制带状物 密度,如 Johanson 模型所示。
According to the model, if the S/D ratio is maintained, a scale-up strategy is to obtain the same Pmax by maintaining the Rf /(W×D) ratio where Rf is the roller force and W is the roller width. The scale-up factor for roller force is calculated by:
根据该模型,如果维持 S/D 比,则放大策略是通过维持 Rf /(W×D)比来得到相同的 Pmax, 此 处 Rf 为轧辊力,W 为轧辊宽。通过如下计算轧辊力的放大系数:
Rf2/Rf1= W2D2/ W1D1
If roller hydraulic pressure is used, it is necessary to obtain the conversion factor between roller hydraulic pressure (bar) to roller force (kN) from the equipment vendor. 如果使用轧辊液压,则必须从设备供应商得到轧辊液压(bar)与轧辊力(kN)间的换算系数。 Alexanderwerk provided the following information:
Alexanderwerk提供了如下信息:
For WP120: 0.0922 kN per cm of roller width for 1 bar roller pressure WP120:轧辊宽0.0922 kN/cm对应于1 bar轧辊压力
For WP200: 0.0869 kN per cm of roller width for 1 bar roller pressure WP200:轧辊宽0.0869 kN/cm对应于1 bar轧辊压力
The scale-up factor for roller pressure was calculated by:
通过如下计算轧辊压力的放大系数:
Rp2/Rp1= 0.0869×D2/ 0.0922×D1
Screw Speed and Roll Speed 螺杆转速和轧辊速度
Assuming no slip at the roller surface in the nip region (i.e., the material is moving at the same speed as the rollers), the mass flow rate (throughput, Q, g/min) of material can be calculated based on mass balance: 假设捏合区的轧辊表面无滑移(即物料以与轧辊相同的速度移动),基于质量平衡可计算物料 的质量流率(生产量,Q,g/min):
Q=ρπDWSNR
where ρ is the ribbon density (g/cc), D is the roller diameter (cm), W is the roller width (cm), S is
the roller gap (cm) and NR is the roller rotation speed (rpm).
此处,ρ 为带状物密度(g/cc),D 为轧辊直径(cm),W 为轧辊宽(cm),S 为轧辊间隙,NR 为 轧辊转速(rpm)。
The powder material is conveyed to the rollers by the screw auger and the mass flow rate is typically proportional to the screw rotation rate: 粉状物料通过螺旋钻头输送到轧辊,质量流率一般与螺杆转速成正比:
Q= CS NS
where, NS is the feed screw rotation speed (rpm) and CS is the amount of material conveyed by
the screw per rotation (g/rotation) which can be determined experimentally.
此处,NS 为螺旋加料器转速(rpm),CS 为每次旋转通过螺杆输送的物料量(g/旋转),这可通 过实验测定。
To achieve the target ribbon density for the given roller gap, the ratio of screw speed to roller speed was maintained constant by setting the two equations for mass flow rate equal to each other as shown below: 为达到给定轧辊间隙的带状物目标密度,螺杆转速与轧辊速度的比应通过设置质量流率彼此 相等的两个方程来维持恒定,如下所示:
NS/NR=ρπDWS/ CS
Mill Screen Orifice Size and Mill Speed 细筛孔径和粉碎机速度
Mill screen orifice size is a scale-independent variable; therefore, it is kept constant upon scale-up. During development, mill speed was not found to be critical for any product quality attributes. In practice, mill speed is set based on first-in first-out principles to avoid ribbon accumulation in the mill. 细筛孔径是与规模无关的变量;因此,一旦放大后,保持恒定。开发中,发现粉碎机速度是 任何产品质量属性的关键。实际上,基于先进先出原则,设置粉碎机速度以避免带状物积聚 在粉碎机中。
Table 52 summarizes the roller compaction and integrated milling process scale-up.
表 52 总结了碾压和集成粉碎工艺的放大。
2.3.6.3 Scale-Up of the Final Blending and Lubrication Process
最终混合和润滑工艺的放大
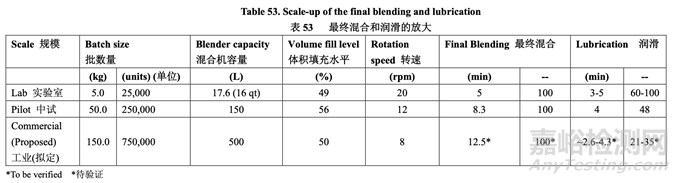
To scale-up the final blending of the granules with talc, the number of revolutions was maintained. 维持转数以放大颗粒和滑石粉的最终混合。
A different strategy was employed to scale-up the final lubrication. Recently, an equation for scaling up the lubrication of a 1:1 MCC:Lactose blend with magnesium stearate was published.16 If the batch size and blender volume of the new process are known, the number of revolutions to be used at the new process condition can be evaluated using the following equation:
不同的策略用于放大最终润滑。最近,公布了一种可放大 1:1 MCC:乳糖的混合物和硬脂酸镁润滑的方程。16 如果已知批数量和新工艺的混合机容量,则 使用如下方程可评估新工艺条件下使用的转数
r2= (V1/3 Fheadspace r)1/(V1/3 Fheadspace r)2
where V is the blender volume, Fheadspace is the headspace fraction (calculated by 100% - fill level %), and r is the number of revolutions. The number of revolutions needed to lubricate the granules with magnesium stearate was calculated based on this equation. The final blending and lubrication process scale-up is summarized in Table 53.
此处,V 为混合机容量,Fheadspace 为顶空部分(由 100%-填充水平%计算),r 为转数。基于该方程计算颗粒和硬脂酸镁润滑所需的转数。表 53 总结了最终 混合和润滑工艺的放大。
2.3.6.4 Scale-Up of the Tablet Compression Process 压片工艺的放大
The same tablet press utilized during the tablet compression process development studies was used for the pilot batch and will be used for commercial scale production. Detailed parameters that affect the tabletting process were already explored and discussed in Section 2.3.5. To increase throughput, all 51 stations were used at the pilot scale successfully and will be used at the commercial scale. The press will be run at the same speed that was studied during development (20-60 rpm). Therefore, dwell time remains unchanged during scale-up.
在压片工艺开发研究中使用的相同的压片机用于中试批并将用于工业规模生产。2.3.5节已考察并讨论了影响压片工艺的详细参数。为增加生产量,中试 规模下成功地使用了所有51个冲模数并将在工业规模下使用。压片机将以开发中研究的相同速度(20~60 rpm)运行。因此,停顿时间在放大中保持不变。
2.3.7 Exhibit Batch 申报批
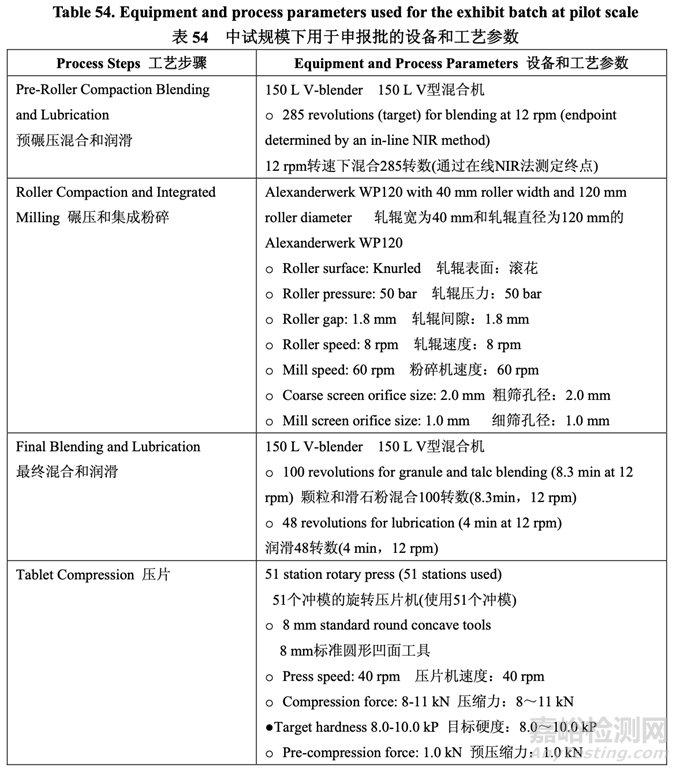
Based on the scale-up principles detailed in Section 2.3.6, a 50.0 kg cGMP exhibit batch was manufactured with drug substance Lot #2 at the pilot scale and the batch was used for the pivotal BE study. Table 54 summarizes the equipment and process parameters used for the exhibit batch at pilot scale.
基于 2.3.6 节中的具体放大原则,在中试规模下使用原料药批号 2 生产了一个 50.0 kg cGMP 的申报批,该批用于关键 BE 研究。表 54 总结了中试规模下用于申报批的设备和工艺参数。
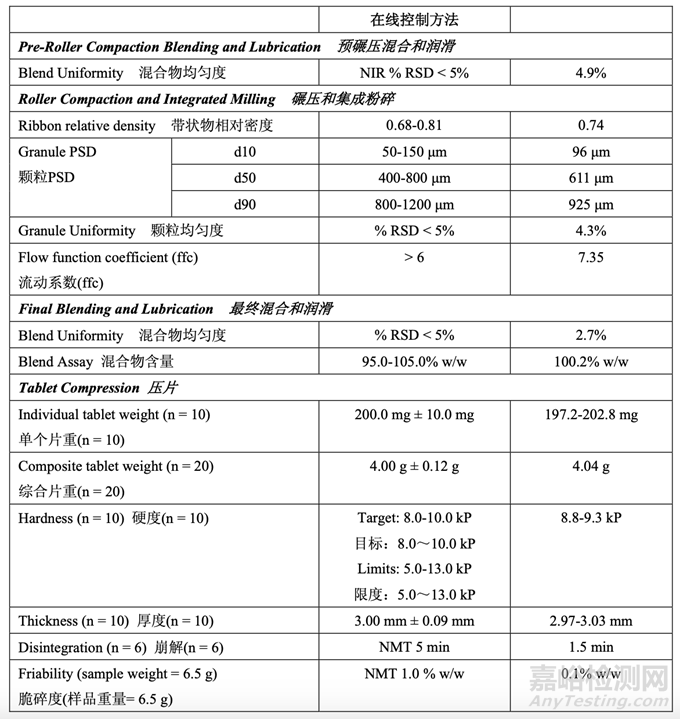
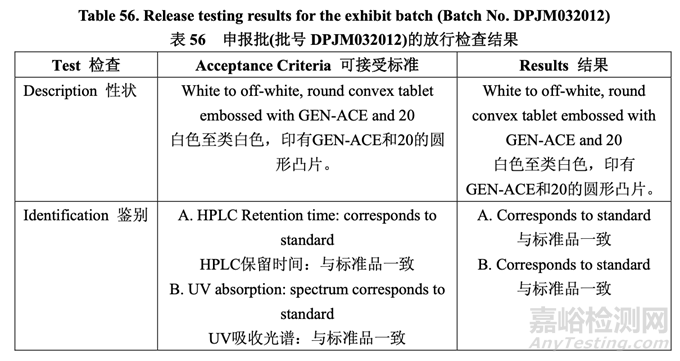
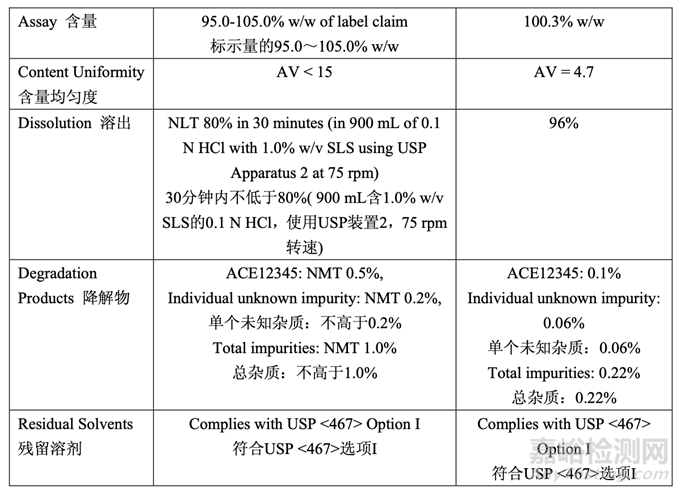
The in-process testing and final release results are summarized in Table 55 and Table 56, respectively.
表 55 和表 56 分别总结了在线检查和最终放行结果。
2.3.8 Updated Risk Assessment of the Drug Product Manufacturing Process
更新的制剂生产工艺的风险评估
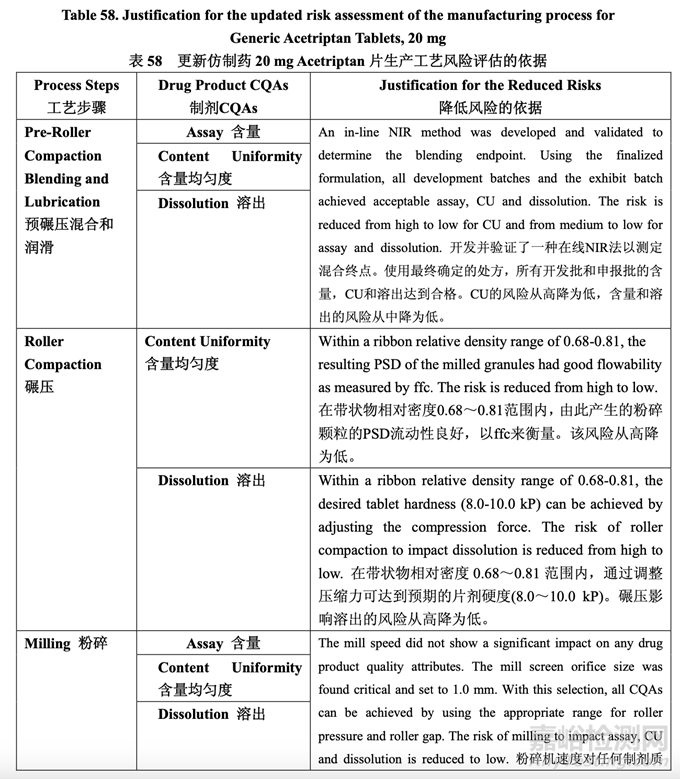
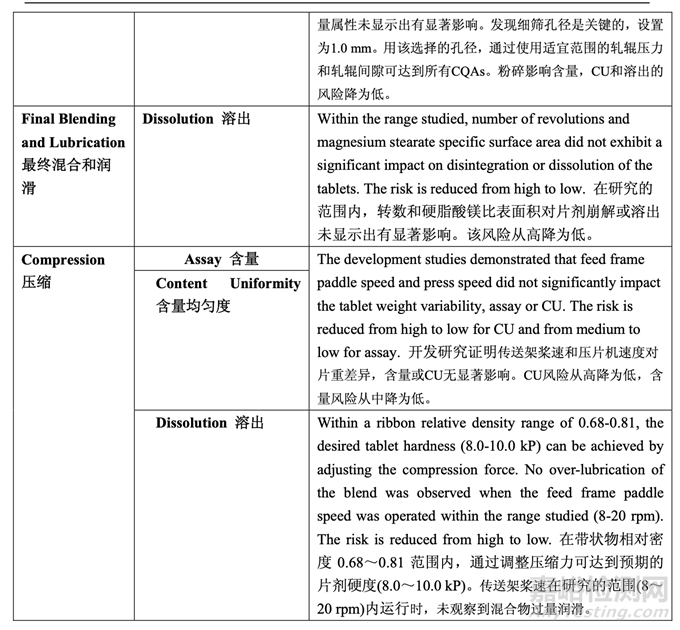
During process development, the identified high risks for each process step were addressed. Experimental studies were defined and executed in order to establish additional scientific knowledge and understanding, to allow appropriate controls to be developed and implemented, and to reduce the risk to an acceptable level. After detailed experimentation, the initial manufacturing process risk assessment was updated in line with the current process understanding. Table 57 presents how the application of the control strategy to the manufacturing process has reduced the identified risks. Table 58 provides the justification for the reduced risk following process development.
工艺开发中,讨论了每个工艺步骤确定的高风险。确定并执行了实验性研究以便建立更多的 科学知识和理解,允许适宜的控制以便开发和执行,并将风险降低至可接受水平。在具体的 实验后,按照目前的工艺理解,更新了初始生产工艺风险评估。表 57 呈现了用于生产工艺 的控制策略怎样降低确定的风险。表 58 提供了降低如下工艺开发风险的依据。
2.4 Container Closure System
容器密封系统
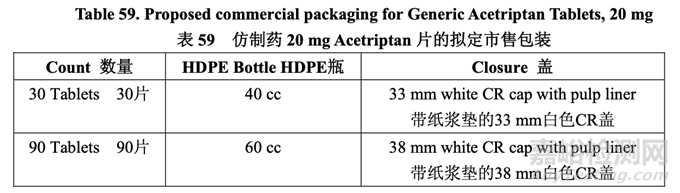
To be consistent with the RLD, the proposed generic drug product is intended to be labeled for storage at 25 °C (77 °F) with excursions permitted to 15-30 °C (59-86 °F). The innovator has chosen round white opaque HDPE bottles with an induction seal liner and child resistant (CR) closure. Generic Acetriptan Tablets, 20 mg, will be similarly packaged and the bottle pack details are summarized in Table 59.
为与 RLD 一致,拟定的仿制药制剂,拟用标签标示贮存在 25 °C (77 °F),允许在 15~30 °C (59~86 °F)内偏离。原研药选择圆形白色不透明 HDPE 瓶,并带有感应密封垫和儿童安全(CR) 盖。仿制药 20 mg Acetriptan 片将有类似的包装,表 59 总结了具体的瓶包装信息。
2.5 Microbiological Attributes 微生物属性
An accelerated stability study of the exhibit batch demonstrated that the drug product has low water activity and is not capable of supporting microbial growth. Routine microbiological testing of Generic Acetriptan Tablets, 20 mg, is unnecessary due to the low water activity of the product and controls on incoming raw materials. 申报批的加速稳定性研究显示制剂的水分活度低,不能支持微生物生长。仿制药 20 mg Acetriptan 片的常规微生物检测是不必要的由于产品具有低水分活度和控制原材料的措施。
2.6 Compatibility 相容性
This section is not applicable because the drug product is a solid oral dosage form and there are no reconstitution diluents.
本节不适用因为制剂是固体口服剂型,无复溶稀释剂。
2.7 Control Strategy 控制策略
Note to Reader: The control strategy is “a planned set of controls, derived from current product and process understanding, that assures process performance and product quality. The controls can include parameters and attributes related to drug substance and drug product materials and components, facility and equipment operating conditions, in-process controls, finished product specifications, and the associated methods and frequency of monitoring and control.”17 致读者:控制策略是“一系列计划的控制措施,来源于目前的产品和工艺理解,确保工艺性 能和产品质量。控制措施可包括与原料药和制剂物料和组分,设备和设施的运行条件,在线 控制,成品质量标准,和相关的方法和监测和控制频率相关的参数和属性。”
The control strategy for Generic Acetriptan Tablets, 20 mg, is built upon the outcome of extensive product and process understanding studies. These studies investigated the material attributes and process parameters that were deemed high risk to the CQAs of the drug product during the initial risk assessment. In some cases, variables considered medium risk were also investigated. Through these systematic studies, the CMAs and CPPs were identified and the acceptable operating ranges were established. All variables ranked as high risk in the initial risk assessment are included in
the control strategy because the conclusion of the experiments was dependant on the range(s) studied and the complex multivariate relationship between variables. Thus, the control strategy is an integrated overview of how quality is assured based on current process and product knowledge. The control strategy may be further refined based on additional experience gained during the commercial lifecycle of the product. However, any post-approval changes should be reported to the agency in accordance with CFR 314.70 and should follow steps as outlined by guidances used for scale-up and post-approval changes.
仿制药20 mg Acetriptan片的控制策略是基于广泛的产品和工艺理解研究的结果。这些研究调 查了被视为初始风险评估中制剂CQAs的高风险的物质属性和工艺参数。在某些情况下,也 调查了视为中风险的变量。通过这些系统研究,确定了CMAs和CPPs并确定了可接受的运行 范围。控制策略包括了所有初始风险评估中列为高风险的变量,因为实验的结论取决于研究 范围和变量间的复杂多元关系。因此,控制策略综合概述了基于目前的工艺和产品知识,怎 样保障质量。基于在产品的商业生命周期中得到的额外经验,可进一步简化控制策略。但是, 根据CFR 314.70,任何批准后的变更应报告给专业的行政结构,并应遵循如用于放大和批准 后变更的指南所概述的步骤。
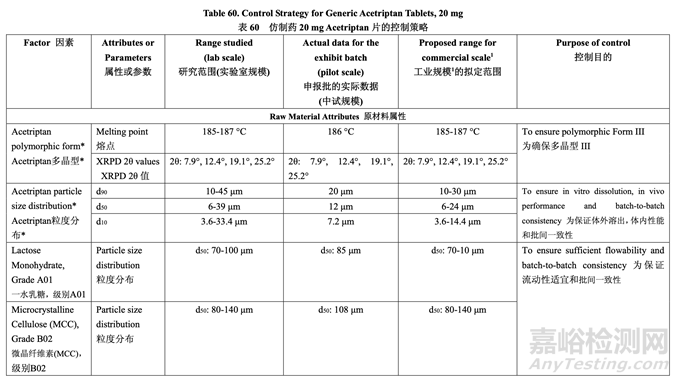
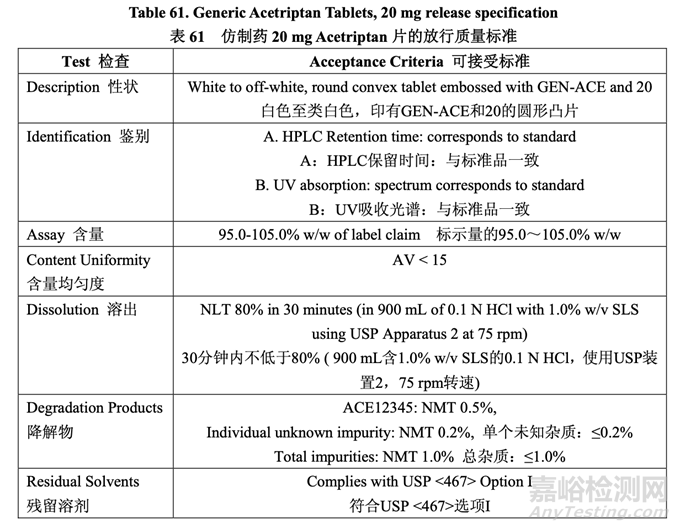
The control strategy for the commercial manufacture of Generic Acetriptan Tablets, 20 mg, is proposed and presented in Table 60. The control strategy includes acetriptan and excipient material attributes to be controlled, in-process controls, high risk process parameter ranges studied during development and the proposed operating ranges for commercial manufacture. The purpose of the controls is also briefly discussed. The release specification for the final product is provided in Table 61.
拟定了仿制药 20 mg Acetriptan 片工业生产的控制策略,见表 60。控制策略包括控制 acetriptan 和辅料的物料属性,在线控制措施,在开发中研究高风险工艺参数范围和拟定工业生产的运 行范围。也简要讨论了控制措施的目的。最终产品的放行质量标准见表 61。
2.7.1 Control Strategy for Raw Material Attributes 原材料属性的控制策略
The drug substance particle size distribution limits arise from a combination of its impact on blending and in vivo performance. The pilot PK study suggested that Generic Acetriptan Tablets, 20 mg, with a drug substance d90 of 30 μm (d50 of 24 μm) or less would be bioequivalent to the RLD. During formulation development, a particle size distribution with a d90 value greater than 14 μm was found to ensure good flow and content uniformity using a fixed blending process. However, implementing a validated in-line NIR method to determine the blending endpoint during process development allowed acceptable blending uniformity and tablet CQAs to be achieved using a drug substance d90 in the range of 10-30 μm.
原料药粒度分布限度由其对混合和体内性能的影响组合而产生。中试PK研究表明仿制药20 mg Acetriptan片,用d90为30 μm (d50为24 μm)或以下的原料药,将生物等效于RLD。处方开 发中,发现粒度分布的d90值应大于14 μm以保证使用固定混合工艺的流动性和含量均匀度良 好。但是,工艺开发中实施了经验证的在线NIR法来判断混合终点,产生的混合均匀度合格, 合格的片剂CQAs将使用d90在10~30 μm范围内的原料药来达到。
Excipient particle size distribution specifications were based on the attributes of the selected grades. For lactose and microcrystalline cellulose, an in-house limit is set on d50 to ensure batch-to-batch consistency.
辅料粒度分布质量标准是基于所选级别的属性。对于 乳糖和微晶纤维素,设置了d50的内部 限度以保证批间一致性。
Based on the analysis of dissolution data collected during formulation development and the results of the pilot PK study, the dissolution medium with 1.0% w/v SLS was more sensitive to product differences than the FDA-recommended method using medium with 2.0% w/v SLS. For thisreason, 1.0% w/v SLS is used in the dissolution medium for the release method in the control strategy.
基于处方开发中采集的溶出数据分析和中试PK研究的结果,含1.0% w/v SLS的溶出介质对 产品差异较使用FDA推荐的方法,即含2.0% w/v SLS的介质更敏感。基于这个原因,在控制 策略的释放方法中,使用含1.0% w/v SLS的溶出介质。
2.7.2 Control Strategy for Pre-Roller Compaction Blending and Lubrication
预碾压混合和润滑的控制策略
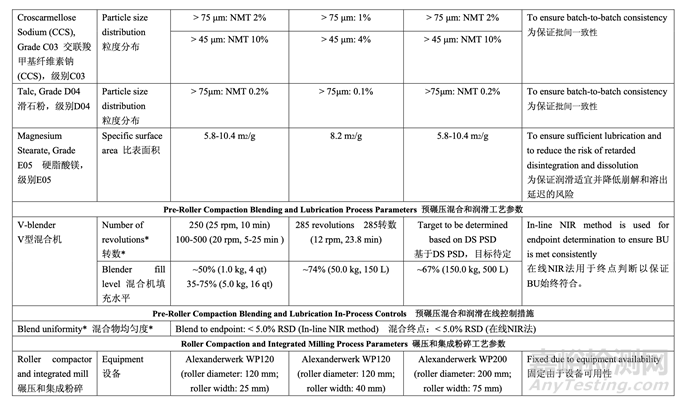
The updated risk assessment (Table 37) for the pre-roller compaction blending and lubrication process step demonstrates that the identified risks to blend uniformity have been reduced by adjusting the number of revolutions to accommodate different acetriptan particle size distributions. A validated in-line NIR method for monitoring the blend uniformity was developed, validated and implemented to terminate the blending based on feedback control when the moving block % RSD of ten consecutive spectra is below 5% for ten consecutive measurements. 更新的预碾压混合和润滑工艺步骤的风险评估(表37)证明通过调整转数以适应acetriptan不 同粒度分布可降低确定为混合物均匀度的风险。基于反馈控制,当10个连续光谱的10个连续 测量值的移动区% RSD低于5%时,开发,验证和实施了一种经验证的用于监测混合物均匀 度的在线NIR法来终止混合。
2.7.3 Control Strategy for Roller Compaction and Integrated Milling
碾压和集成粉碎的控制策略
The intent of the control strategy for roller compaction is to maintain the ribbon density within the required range to ensure drug product CQAs are met. To maintain a ribbon relative density of 0.68-0.81 during routine operation, the roller pressure and roller gap will be controlled. The ribbon density will be monitored as an in-process control during roller compaction. 碾压的控制策略目的是维持带状物密度在要求的范围内以保证制剂CQAs符合。为维持常规 运行中的带状物相对密度在0.68~0.81,将控制轧辊压力和轧辊间隙。将监测带状物密度作 为碾压中的一种在线控制措施。
For milling, the mill screen orifice size (1.0 mm) was selected to ensure that the granule size distribution remains within the acceptable range. The acceptable range for mill speed (20-100 rpm) was established and can be adjusted within the range to accommodate different throughput from the roller compaction step. If a change to the mill screen orifice size is made (e.g., increase or decrease) then the impact on granule size distribution and assay of sieve cuts will be reassessed across the pre-defined ribbon density range.
对于粉碎,选择了细筛孔径(1.0 mm)以保证颗粒粒度分布保持在合格范围内。确定了粉碎机 速度的合格范围(20~100 rpm)并在范围内进行调整以适应来自碾压步骤的不同生产量。如果 变更了细筛孔径(如增加或减少),那么在整个预定义的带状物密度范围内,将重新评估对颗 粒粒度分布和筛分颗粒含量的影响。
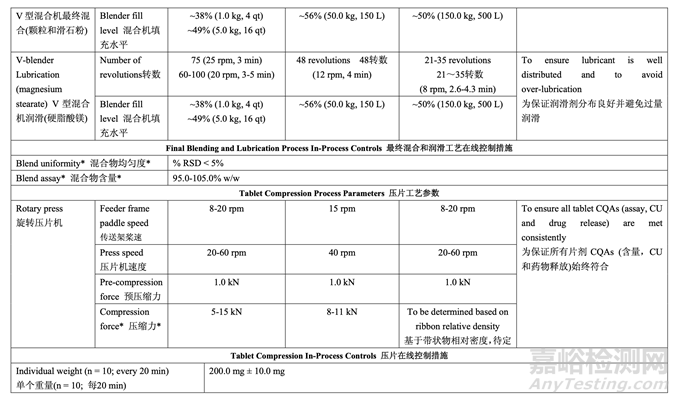
2.7.4 Control Strategy for Final Blending and Lubrication 最终混合和润滑的控制策略
The control strategy for blending the granules with talc is to maintain the targeted number of revolutions. For the granule lubrication with magnesium stearate, the control strategy is to adjust the number of revolutions based on the blender capacity used (headspace) and the volume of the V-blender according to the scientific literature. 颗粒和滑石粉混合的控制策略是维持规定的目标转数。对于颗粒与硬脂酸镁的润滑,根据科 学文献,控制策略是调整转数,基于使用的混合机容量(顶空)和V型混合机的容量。
2.7.5 Control Strategy for Tablet Compression 压片的控制策略
The control strategy for compression is to maintain the in-process tablet attributes of weight, hardness, thickness, friability and disintegration within the required ranges. The fill cam below the die table adjusts the lower punch to the appropriate height to control fill depth and ultimately tablet weight. The target compression force required to produce tablets with the desired hardness, and ultimately friability and disintegration, is established at the beginning of each run. After tablets with the target weight and hardness are obtained during the tablet press set-up, the upper punch penetration depth and the fill depth are fixed. The compression force is continuously measured throughout the run for each tablet and compared to the target compression force. The main compression height is automatically adjusted to keep the average force as close as possible to the target set point. Upper and lower limits of compression force are set and any tablet that registers a compression force outside these limits is automatically rejected by the tablet press.
压缩的控制策略是维持重量、硬度、厚度、脆碎度和崩解的在线片剂属性在要求的范围内。 填料凸轮低于冲台,则调整下冲至适宜的高度以控制填料深度并最终控制片重。在每次运行 的开始确定了可生产出具有预期硬度并最终具有预期脆碎度和崩解片剂的目标压缩力。在压 片机调整中得到了具有目标重量和硬度的片剂后,固定上冲穿透深度和填料深度。这个运行 中连续测量每片的压缩力并与目标压缩力比较。自动调整主压缩高度以保持平均力尽可能接 近于目标设定值。设置压缩力的上限和下限,由压片机自动去除显示的压缩力超出这些限度 的任何片剂。
2.7.6 Product Lifecycle Management and Continual Improvement
产品生命周期管理和持续改进
Upon approval, the manufacturing process for Generic Acetriptan Tablets, 20 mg, will be validated using the lifecycle approach that employs risk-based decision making throughout the drug product lifecycle as defined in the FDA process validation guidance.18 经批准,使用生命周期法来验证仿制药 20 mg Acetriptan 片的生产工艺,即在整个制剂生命 周期内使用如 FDA 工艺验证指南所定义的基于风险的决策。18
The QbD approach taken during pharmaceutical development of Generic Acetriptan Tablets, 20 mg, facilitated product and process understanding relevant to Stage 1 (Process Design) of process validation. During Stage 1, the commercial manufacturing process was defined based on knowledge gained through development and scale up activities and a strategy for process control was developed. The goal of Stage 2 (Process Qualification) is to evaluate if the process is capable of reproducible commercial manufacturing. The manufacturing facility will be designed according to cGMP regulations on Building and Facilities.19 Activities will be taken to demonstrate that utilities and equipment are suitable for their intended use and perform properly. The protocol for process performance qualification will be written, reviewed, approved, and then executed to demonstrate that the commercial manufacturing process performs as expected. The goal of Stage 3 (Continued Process Verification) is continual assurance that the process remains in a state of control (the validated state) during commercial manufacture.
仿制药 20 mg Acetriptan 片的药物开发期间采用的 QbD 法,有助于理解与工艺验证阶段 1(工 艺设计)相关的产品和工艺。在阶段 1 中,基于从开发和放大活动中得到的知识,确定了工 业生产工艺并开发了一种工艺控制的策略。阶段 2(工艺评定)的目标是评估工艺是否能重现 工业生产。将根据关于建筑和设备的 cGMP 法规来设计生产设备。19 将采取行动以证明设施 和设备适用于其预期用途并正确操作。将编写,审核,批准并执行工艺性能确认方案以证明 工业生产工艺如预期那样进行。阶段 3(持续的工艺验证)的目标是持续保证在工业生产中, 工艺保持在控制状态(经验证的状态)。
Throughout the product lifecycle, the manufacturing process performance will be monitored to ensure that it is working as anticipated to deliver the product with desired quality attributes. Process stability and process capability will be measured and evaluated. If any unexpected process variability is detected, appropriate actions will be taken to correct, anticipate, and prevent future problems so that the process remains in control. The additional knowledge gained during routine manufacturing will be utilized for adjustment of process parameters as part of the continual improvement of the drug product. As a commitment, the regulatory agency will be notified in accordance with CFR 314.70 regarding each change in each condition beyond the variability already provided in this application.
整个产品生命周期中,将监测生产工艺性能以确保正在如预期的那样工作以提供预期质量属 性的产品。将测量并评估工艺稳定性和工艺能力。如果检测到任何非预期的工艺变异性,则 将采取适宜的措施以校正,预期和预防将来的问题以便工艺保持在控制中。常规生产中得到 的额外知识将用于调整工艺参数作为制剂持续改进的一部分。作为承诺,根据 CFR 314.70 关于每种情况下的各个变更超过了该申请中已提供的变异性,将通知监管机构。
参考文献:
Example QbD IR Tablet Module 3 Quality 3.2.P.2 Pharmaceutical Development,FDA,2012.