您当前的位置:检测资讯 > 生产品管
嘉峪检测网 2024-08-13 20:01
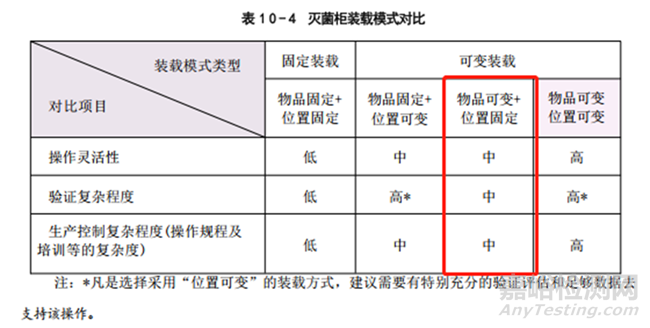
下图中这里所谓的物品可变,更多的实践是灭菌物品数量可变(灭菌物品本身以及摆放位置不可变),今天分享的是PDA Journal上的一些思路。
Flexible Loading Pattern Approach in Overkill Steam Sterilization Based on the Physical Properties of Steam and Thermodynamics of Sterilization
基于蒸汽的物理特性和灭菌热力学的过度杀菌蒸汽灭菌中的灵活装载模式方法。
ABSTRACT: Because overkill steam sterilization processes in autoclaves are considered critical, they are highly scrutinized, and the use of autoclaves in fixed loading patterns is a common approach to the interpretation of regulatory requirements. Many such regulations are attributed to tradition and a buildup of restrictions that aim to improve the levels of assurance of the process and minimize risk. However, these measures complicate the operation and qualification of autoclaves, becoming cumbersome, time-consuming, and costly. In actuality, overkill sterilization is one of several processes in the pharmaceutical industry that provides the highest levels of sterility assurance. This method provides a minimum reduction of highly durable spore populations of 12 logs, achieving a probability of a nonsterile unit (PNSU) of 10-6. The physical properties of steam and the thermodynamics of steam sterilization constitute a predictable and repeatable process that can be monitored and verified. The high assurance level of overkill sterilization, combined with the properties of steam, actualizes a high safety margin that encompasses nearly every load type and load configuration when the cycle is performed under certain basic rules. The aim of this article is to present data that advocate and favor an approach that allows greater freedom and variability in arranging items in an autoclave when running overkill cycles, without the need to qualify each configuration.
摘要:因为高压蒸汽灭菌过程在灭菌器中被视为关键过程,它们受到严格的审查,使用固定装载模式的灭菌器是解释监管要求的常见方法。许多这样的规定归因于传统和限制的积累,旨在提高过程的保证水平并将风险降至最低。然而,这些措施使灭菌器的操作和确认复杂化,变得笨重,耗时,昂贵。实际上,过度杀灭法是制药工业中提供最高无菌保证水平的几个过程之一。这种方法至少能将非常耐用的孢子群体减少12个logs,从而实现非无菌单元(PNSU)的概率为10−6。蒸汽的物理性质蒸汽灭菌的热力学构成了一个可预测和可重复的过程,可以监控和验证。过度杀菌的高保证水平结合蒸汽的特性,在遵循一定基本原则的情况下,实现了一个高安全边际,几乎涵盖了所有类型的装载和装载配置。本文的目的是提出数据,支持并倾向于一种方法,该方法在运行过度杀菌周期时,允许在高压蒸汽灭菌器内安排物品时有更大的自由度和可变性,无需对每种配置进行验证。
1、在安全边际的更安全一侧
On the Safe Side of Safe Side
Overkill Cycles 过度杀灭周期
Steam sterilization is one of the most common and important practices in parenteral manufacturing, bound by many rules and regulations. In a routine inspection, the validation, maintenance, and operation of an autoclave are usually examined thoroughly. Without downgrading the criticality of steam sterilization, many requirements are derived from the definition of “critical”, even when there is no scientific basis for the requirements. In other cases, the requirements are extended far beyond what is really needed to be “on the safe side”.
蒸汽灭菌是输液制造中一种最常见且重要的实践,受到许多规则和法规的约束。在常规检查中,通常会对高压蒸汽灭菌器的验证、维护和操作进行彻底的检查。在不降低蒸汽灭菌关键性的前提下,许多要求源自“关键”一词的定义,即使这些要求没有科学依据。在其他情况下,为了“更安全”,要求被扩展到了真正需要的范围之外。
The rationale of the overkill cycle was that it would allow more flexibility in sterilization, due to the high safety margins that it provides to compensate for potential variation in this process. The premise of overkill cycles is that the bioburden population and its resistance can be ignored, because they are irrelevant to the outcome. This genuine benefit is eliminated, because in actuality, overkill sterilization cycles are restricted by additional regulatory requirements and “expectations” to inflate the safety margin. The requirement for fixed loading patterns is one of the most burdensome of these principles.
过度杀菌周期的原理是,由于它提供了很高的安全边际来弥补过程中可能出现的变化,因此它将允许在灭菌中有更多的灵活性。过度杀菌周期的前提是,可以忽略生物负荷的种群数量和其抗性,因为这些与结果无关。这种真正的好处被消除了,因为在现实中,过度杀菌周期受到额外的监管要求和“期望”的限制,这些要求和期望增加了安全边际。固定装载模式的要求是这些原则中最繁重的一个。
2、固定装载模式的要求
Requirements of Fixed Loading Patterns
In a fixed loading pattern, the items that are to be sterilized are arranged according to a scheme or depiction that shows their exact number, size, position, and orientation in the chamber. This arrangement is validated, and if all the acceptance criteria are met, the load is approved for production. If a new item is added to a validated load, the load is considered to be new, and the validation must be repeated. The same process applies to items that have been validated in other loads and, due to changing production requirements,must now be sterilized with other items in a different load
在固定装载模式中,待灭菌的物品根据方案或图示所展示的它们的确切数量、大小、位置和方向在腔室内进行排列。这种排列方式经过验证,一旦满足所有接受标准,装载量就会被批准用于生产。如果向经过验证的装载量中添加了新物品,则该装载量被视为新的装载量,必须重新进行验证。同样的过程也适用于那些在其他装载量中已经经过验证的物品,由于生产需求的变化,现在必须与其他物品一起在不同的装载量中进行灭菌。
The roots of this method date back to the 1970s, when many practices that were related to large-volume parenteral (LVP) sterilization were universally adopted by the small-volume parenteral (SVP) industry, regardless of their use in a different setting (5). At that time, when gravity displacement autoclaves were used in many facilities, this principle was justified. Since then, autoclave design has evolved considerably, with the advent of vacuum and steam pulses to remove air from porous loads and greater control over temperature and pressure in the chamber. In contrast, validation practices have remained almost unchanged.
这种方法的根源可以追溯到20世纪70年代,当时许多与大容量肠外(LVP)灭菌相关的实践被小容量肠外(SVP)行业普遍采纳,而不管它们在不同设置中的使用情况。当时,许多设施使用的是依靠重力置换的灭菌器,这一原理在当时是合理的。从那时起,随着真空和蒸汽脉冲技术的出现,用于从多孔负载中去除空气,以及对腔室内温度和压力的更严格控制,灭菌器的设计已经发生了显著的演变。相比之下,验证实践几乎没有任何变化。
In certain firms, only exact, validated loads are allowed to be run—if for example, just 1 of the 20 items that are validated in the full load is needed, the entire load must be prepared (i.e., washed, bagged, and arranged) and sterilized. To this end, some firms use maximal and minimal load cycles (Max-Min approach), which provides more flexibility. But, when a new item isrequired or validated loads must be combined, the new load needs to be validated again before use
在某些公司,只允许运行确切经过验证的装载量——例如,如果只需要运行已在完整装载量中验证的20个项目中的1个,那么必须准备整个装载量(即,洗涤、包装并排列)并进行灭菌。为此,一些公司使用最大和最小装载周期(最大-最小方法),这提供了更多的灵活性。但是,当需要一个新的项目或必须组合经过验证的装载量时,新的装载量在使用前需要重新验证。
The requirement for fixed loading patterns is triggered by the belief that there is an interaction between items, that one item can influence or interact with an adjacent item, and that the item per se might not be sterilized in a different area in the autoclave—that is, that items can “steal” steam from other items, creating a cold spot or restricting steam flow to other items. For example,heavy items could be believed to be “steam-consuming” items that reduce the supply of steam to other items. This suspicion then leads to the requirement that many loading patterns be validated and maintained through revalidation activities.
固定装载模式的要求源于这样一种信念:物品之间存在相互作用,一个物品可能会影响到相邻物品,或者物品本身在高压蒸汽灭菌器的不同区域可能不会被灭菌——也就是说,物品可能会从其他物品那里“抢夺”蒸汽,从而制造出冷点或限制蒸汽流向其他物品。例如,人们可能认为重量较大的物品是“消耗蒸汽”的物品,它们减少了对其他物品的蒸汽供应。这种怀疑随后导致了要求对许多装载模式进行验证,并通过对重新验证活动来维持。
The standard validation procedure that is used by certain firms is to run three distribution cycles and three penetration cycles for each new load, rendering the workload and downtime of the facility to be significant and costly. Reducing nonvalue-added validation activities can create significant savings for facilities, with no adverse impact on patient safety.
某些公司使用的标准验证程序是对每个新装载量运行三个分布周期和三个穿透周期,这使得设施的工作量和停机时间都相当大且成本高昂。减少不增加价值的验证活动可以为设施创造显著的节省,且不会对患者安全产生负面影响。
This article will attempt to challenge this difficult question with data and convincing arguments, suggesting an approach that allows greater freedom and variability in arranging items in an autoclave when running overkill cycles, without the need to qualify each configuration or any adverse impact on patient safety or the required sterility assurance levels.
这篇文章将尝试用数据和有说服力的论点挑战这个难题,提出一种方法,允许在运行过度杀菌周期时,在高压蒸汽灭菌器内安排物品时有更大的自由度和可变性,而无需对每个配置进行验证,也不会对患者安全或所需的无菌保证水平产生任何负面影响。
3、饱和蒸汽的物理性质和灭菌热力学
Physical Properties of Pure Saturated Steam and the Thermodynamics of Sterilization
Steam sterilization relies on the physical properties of pure saturated steam and the thermodynamics of the sterilization process. Any claim that relates to the ability of steam to sterilize should be evaluated in light of its properties, as described following.
蒸汽灭菌依赖于饱和纯蒸汽的物理特性和灭菌过程的热力学原理。任何与蒸汽灭菌能力相关的主张都应该基于其特性来评估,具体特性如下
1. Steam releases a large amount of energy relative to its weight when it condenses. A small amount of steam can heat a large load, as well as the metal structure of the autoclave. As it condenses, 1 kg of saturated steam releases 2200 kJ, which, when condensed, can heat over 40 kg of stainless steel (SS) from 20˚C to 121˚C ; thus, heating “heavy” metal items does not require large amounts of steam.
1.蒸汽在凝结时相对于其重量释放出大量的能量。少量的蒸汽就能加热大量的负载,包括灭菌器的金属结构。当1公斤的饱和蒸汽凝结时,它释放出2200千焦的能量,这足以将40公斤以上的不锈钢从20 ˚C加热到121 ˚C;因此,加热“重”金属物品并不需要大量的蒸汽。
2. When saturated steam is condensed, its volume decreases significantly (1 L of saturated steam turns into approximately 1.2 mL of condensate) .
Whenever condensation occurs (when steam makes contact with cold areas and condenses), the reduction in volume will bring more steam to cold sections due to the decrease in pressure until the item reaches the same temperature as the steam and no further heating is needed.
2.当饱和蒸汽凝结时,其体积显著减少(1升饱和蒸汽大约转变为1.2毫升的凝结水)。每当发生凝结(即蒸汽接触到冷的区域并凝结时),由于压力降低,体积的减少会将更多蒸汽带到冷的区域,直到物品加热到与蒸汽相同的温度,此时不再需要进一步加热。这一过程有助于确保蒸汽灭菌过程中所有物品都能均匀地达到灭菌所需的温度。
3. There is a 1:1 correlation between saturated steam pressure and its temperature in an enclosed space with constant volume. After the pressure stabilizes, temperatures in the chamber become steady and will be maintained throughout the chamber as a result of the stabilization of pressure.
3.在恒定体积的封闭空间内,饱和蒸汽的压力与其温度存在1:1的相关性。压力稳定后,腔室内的温度变得稳定,并且由于压力的稳定,腔室内的温度将保持一致。
4. Steam that is under pressure in an enclosed space does not flow like air; thus, there are no steam flow patterns in the autoclave. Items do not obstruct steam or prevent it from entering other objects. Providing a load does not block the passage of steam completely—steam will reach all items. As indicated in Point 2, whenever condensation arises, the steam that is nearest to the condensation point will move toward it. These movements occur on a molecular level and are thus rapid; consequently, steam molecules are not influenced by the other items. Combining this inherent property of steam with the property in Point 3 ensures that wherever items are positioned in the chamber, each element will be subjected to the same sterilization conditions. The only interaction that might occur between items is the condensate, which can drip from heavy nonporous items onto lower items; this situation can be resolved easily by placing the former on the lower shelf of the autoclave.
4.在封闭空间中加压的蒸汽不会像空气那样流动;因此,在高压蒸汽灭菌器内不存在蒸汽流动模式。物品不会阻碍蒸汽或阻止其进入其他物体。即使装载了物品,也不会完全阻塞蒸汽的通道——蒸汽能够到达所有物品。如第2点所述,每当发生凝结时,最接近凝结点的蒸汽会向其移动。这些运动发生在分子层面上,因此速度很快;因此,蒸汽分子不会受到其他物品的影响。结合蒸汽的这一固有属性和第3点所述的属性,可以确保无论物品在腔室内如何定位,每个元素都将受到相同的灭菌条件。物品之间可能发生的唯一交互是凝结水,它可能从重量较重的非多孔物品滴落到下方的物品上;通过将前者放置在高压蒸汽灭菌器的下层架上,可以轻松解决这种情况。
5. Heat can be transferred between objects only when there is a difference in temperature. Once the items in the autoclave attain the temperature of the steam, no more energy is transferred to the item, and further condensation stops—an equilibrium is reached. Additional small quantities of steam enter the autoclave only to compensate for the heat loss to the doors and chamber walls (even when the jacket is heated, because the temperature of the jacket is lower than that of the chamber).
5.只有在存在温度差时,物体之间才能传递热量。一旦灭菌器内的物品达到蒸汽的温度,就不会再向物品传递更多的能量,进一步的凝结就会停止——达到了平衡。少量的蒸汽进入灭菌器只是为了补偿对门和腔室壁的热损失(即使灭菌器的外层被加热,因为外层的温度仍然低于腔室的温度,腔室内的蒸汽仍需补充,以确保灭菌过程中温度的均匀性和一致性)。
6. After an equilibrium is reached (after the heating phase and at the outset of the sterilization phase), pressure across the chamber will be equal, according to Pascal’s law.
6.达到平衡后(在加热阶段之后和灭菌阶段开始时),根据帕斯卡定律,腔室内的压力将是均匀的。
7. Steam and air (primarily nitrogen and oxygen) are small molecules (>1 nm); thus, air removal and steam penetration can also be achieved through narrow channels or gaps. Large quantities of steam require wider passageways, but even with small gaps, steam will eventually penetrate all parts of an object.
7.蒸汽和空气(主要是氮气和氧气)都是小分子(直径大于1纳米);因此,即使通过狭窄的通道或缝隙,也能实现空气的移除和蒸汽的渗透。大量的蒸汽需要更宽的通道,但即使存在小的缝隙,蒸汽最终也会渗透到物体的所有部分。
With regard to load, other physical properties are involved in steam sterilization. Table I lists the load properties of items and their effects on sterilization. Combining these properties simplifies the decision over which items are easy or more challenging to sterilize. Table II lists examples of common items. Note that the table merely compares items and does not definitively state whether an item is challenging for an overkill cycle.
在蒸汽灭菌中,与装载量相关的其他物理属性也起着重要作用。表I列出了物品的装载属性及其对灭菌的影响。结合这些属性可以简化判断哪些物品容易或更具挑战性进行灭菌的决策过程。表II列出了一些常见物品的例子。需要注意的是,该表仅比较了物品,并没有明确指出某个物品对于过度杀菌周期是否具有挑战性
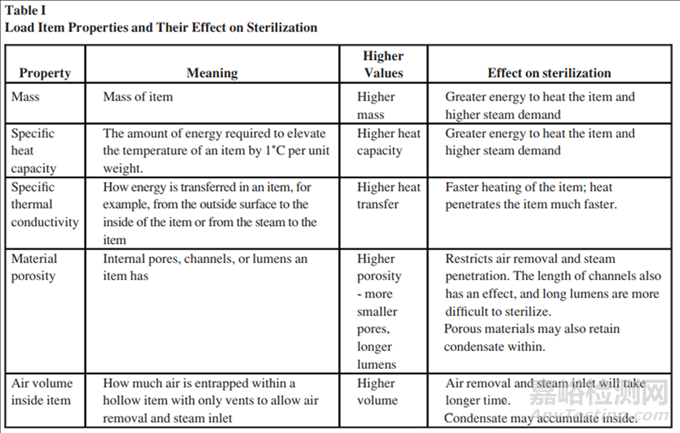
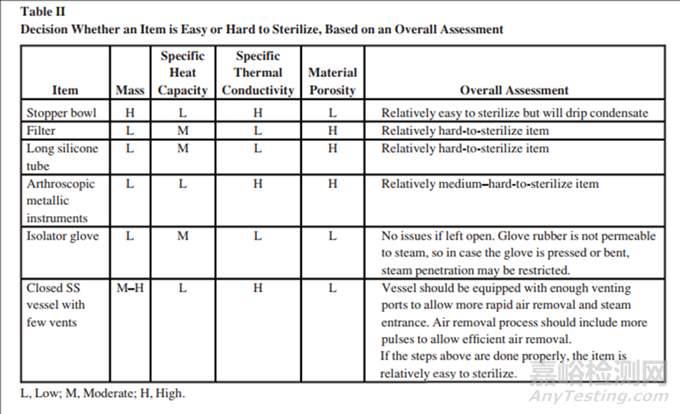
In summary:总结如下
1. Steam can heat a heavy load at low quantities due to high latent heat of condensation (Point 1). Fresh steam will fill the low-pressure zones that are created due to condensation (Point 2), supplying more energy. Heavy loads might elongate the heating phase, but this extra time is not included in the sterilization phase time. Eventually, after the load reaches the sterilization temperature, there is no difference between heavy and light loads, because there is no further transfer of energy to the load (Point 5). Mass should not be the chief parameter for selecting challenging items. The results of the tests in Pavell and Hughes (9) also showed that the more challenging items were the filter and the cleaning hose with a valve on one end, neither of which was among the heaviest items.
1.蒸汽的高凝结潜热使其即使在数量较少的情况下也能加热质量大的负载。新鲜的蒸汽将填补由于凝结而产生的低压区域,提供更多的能量。沉重的负载可能会延长加热阶段,但这个额外的时间不包括在灭菌阶段的时间之内。最终,在负载达到灭菌温度后,重负载和轻负载之间没有区别,因为对负载没有进一步的能量传递。质量不应是选择具有挑战性物品的主要参数。Pavell和Hughes的测试结果也表明,更具挑战性的物品是过滤器和一端带阀门的清洁软管,这两者都不是最重的物品。
2. One of the most common myths that concern steam sterilization is that the high lethality of steam is attributed to the high energy transfer to the sterilized object during condensation (13): “Direct steam contact with the surface of the object to be sterilized is required for the steam to transfer its stored energy to the object.” Direct contact is definitely required, because the moisture in the steam must make contact with the item, but the last section of this statement becomes irrelevant when the setpoint temperature has been attained by all load items. As indicated in Point 5, energy transfer occurs only during the heating phase; throughout the sterilization phase, there is no energy transfer, and microorganisms are eradicated by maintaining the temperature and water molecules contact for the required time
2.关于蒸汽灭菌的一个常见误解是,蒸汽的高致命性归因于在凝结过程中对被灭菌物体的高能量传递:“蒸汽必须与被灭菌物体的表面直接接触,才能将其储存的能量传递给物体。” 直接接触绝对是必需的,因为蒸汽中的水分必须与物品接触,但当所有负载项目达到设定温度时,这一说法的后半部分就变得无关紧要了。能量传递仅在加热阶段发生;在整个灭菌阶段,没有能量传递,微生物是通过维持温度和水分子接触所需时间来根除的。
3. From Points 2, 4, 6, and 7, I conclude that there is no impact of the location of items in the autoclave with regard to achieving the same extent of sterilization. The only parameter that requires attention relates to the prevention of condensate dripping. This conclusion was also the outcome of Pavell and Hughes (9)
3.从观点2、4、6和7,我得出结论,物品在灭菌器中的位置对于实现相同程度的灭菌没有影响。唯一需要关注的参数是防止凝结水滴落。这也是Pavell和Hughes研究的结论。
4、空气去除和蒸汽渗透
Air Removal and Steam Penetration
Steam sterilization is based on the use of pure steam. When air or other noncondensable gases (NCGs) are present, they will affect the sterilization on several levels
蒸汽灭菌基于使用纯蒸汽。当存在空气或其他不可冷凝气体(NCGs)时,它们会在多个层面上影响灭菌效果
1. Steam will follow the physical properties described above only when it is pure. When it is impure, the outcome is not predicted as easily.
1.只有纯蒸汽才会遵循上述描述的物理属性。当它不纯净时,结果不容易预测
2. Effective steam sterilization is predicated on the steam being in contact with the item. NCGs create isolation and can limit the destructive effectiveness
of steam.
2.有效的蒸汽灭菌基于蒸汽与物品的接触。不可冷凝气体(NCGs)会造成隔离,可能限制蒸汽的破坏性效果。
3. Steam lethality is attributed to the presence of water molecules. NCGs reduce steam lethality because they do not include water molecules.
3.蒸汽的致命性归因于水分子的存在。不可冷凝气体降低了蒸汽的致命性,因为它们不包含水分子。
Steam purity is influenced by how steam is produced and transferred and by efficient air removal from the chamber and load. Assuming that steam is tested on a regular basis for low levels of NCGs2, proper air removal should be ensured before starting the sterilization phase. In most cases, air removal is more important than additional sterilization time and increased temperature. The PNSU calculations in the first section are valid only with efficient air removal so that the full lethality of steam is applied.
蒸汽的纯度受其产生和转移的方式以及从腔室和负载中有效去除空气的影响。假设蒸汽会定期检测低水平的不可冷凝气体(NCGs),那么在开始灭菌阶段之前应确保适当的空气去除。在大多数情况下,空气去除比增加灭菌时间和提高温度更为重要。第一部分中的PNSU计算仅在有效去除空气的情况下才有效,以便发挥蒸汽的全部致命性。
Air removal and steam penetration are complementary, because the same channel that allows air to be removed from internal voids allows steam to enter these voids.
空气去除和蒸汽渗透是相辅相成的,因为允许空气从内部空隙中排出的相同通道也允许蒸汽进入这些空隙。
The level at which air is not considered to be harmful depends on the load. Small air bubbles that are dissolved in steam are not a problem, but their accumulation in closed packs or pockets can become an issue(16).
空气无害的水平取决于负载。溶解在蒸汽中的小气泡不是问题,但如果它们在封闭的包装或口袋里积聚,可能会成为问题。
Effective air removal and steam penetration are achieved by the repeated evacuation and entrance of steam into the voids. Collectively, this process entails dilution of the air in steam and subsequent extrusion of the mixture. The efficacy of the process is dictated by two factors:
通过反复抽出和进入蒸汽到空隙中实现有效的空气去除和蒸汽渗透。总的来说,这个过程包括蒸汽中空气的稀释及其后混合物的挤出。这个过程的效果由两个因素决定:
1.Values of pressure for the vacuum and steam pulses
1.真空和蒸汽脉冲的压力值。
2. Pulse time. Slow pulses are more effective in removing air from challenging items, such as long lumens, porous loads, and packed items, because physical restrictions slow the evacuation and steam penetration. This property is the chief reason that a minimal load is required in an overkill cycle. In minimal loads, the evacuation time and steam pulse are shorter, impeding the air removal from and steam penetration of challenging items. In certain autoclaves, pulses duration can be set and be equal, regardless of load size, but in many autoclaves, this option does not exist. In such cases, this issue can be overcome through redundancy by adding more pulses or increasing the difference between the vacuum and steam pressures, rendering even shorter pulses sufficiently effective.
2.脉冲时间。慢速脉冲在去除具有挑战性的物品中的空气方面更有效,例如长通道、多孔负载和包装紧密的物品,因为物理限制减慢了抽气和蒸汽渗透的速度。这种特性是过度杀菌周期中需要最小负载的主要原因。在最小负载中,抽气时间和蒸汽脉冲时间较短,阻碍了从具有挑战性的物品中去除空气和蒸汽的渗透。在某些灭菌器中,可以设置脉冲持续时间并使其相等,不管负载大小如何,但在许多灭菌器中,并不提供这种选项。在这种情况下,可以通过增加脉冲次数或增加真空和蒸汽压力之间的差异来实现冗余,使即使是更短的脉冲也足够有效。
In an overkill cycle, air removal is the main parameter when determining whether an item is simple or difficult to sterilize. When air removal is conducted properly, steam will penetrate all voids, eliminating “hard-tosterilize or challenging items.” Item Preparation, Wrapping, and Orientation Preparation, wrapping, and orientation are significant factors in the success of the sterilization cycle, because they impact air removal, steam penetration, and condensate removal (8). An item that is validated after being prepared, wrapped, and oriented in the chamber according to specific instructions should always be sterilized in the manner that it was validated. For example, a tube coiling of a specific length should be inserted into a double bag and placed flat on the autoclave shelf. A validated item includes its preparation, wrapping, and orientation.
在过度杀菌周期中,空气去除是决定物品是简单还是难以灭菌的主要参数。当空气去除进行得当,蒸汽将渗透到所有空隙中,消除了“难以灭菌或具有挑战性的物品”。物品的准备、包装和方向 准备、包装和方向是灭菌周期成功的重要因素,因为它们影响空气的去除、蒸汽的渗透和凝结水的排出。根据特定说明在腔室中准备好、包装好并定位好的物品应该始终按照其经过验证的方式进行灭菌。例如,特定长度的管圈应插入双层袋中,并平放在灭菌器架上。经过验证的物品包括其准备、包装和方向。
Data Collection and Interpretation Data were collected from actual validation runs that were performed at a specific facility from 2017 to 2019, as presented in Table III. The data are used to assess differences between maximal and minimal load results.
数据收集与解释:数据收集自2017年至2019年在特定设施进行的实际验证运行,如表III所示。这些数据被用来评估最大装载量和最小装载量结果之间的差异
The maximal load is prepared by collecting the more challenging items (items with a minimal F0 value) from all validated loadings.
最大装载量是通过从所有经过验证的装载中收集更具挑战性的物品(即具有最小F0值的物品)来准备的。
The minimal load usually includes one item that is found to be more challenging during the run of the maximal load
最小装载量通常包括在最大装载量运行过程中发现的更具挑战性的一个物品。
Data were collected from 数据收集自:
6 autoclaves, varying in size and age
6台不同尺寸和使用年限的高压蒸汽灭菌器
4 types of loads—garments, gloves, tools, and rubber stoppers
4种不同类型的装载物品:服装、手套、工具和橡胶塞
4 types of air removal processes
4种不同的空气去除过程
Cycles from 2017, 2018, and 2019
2017年、2018年和2019年的循环数据
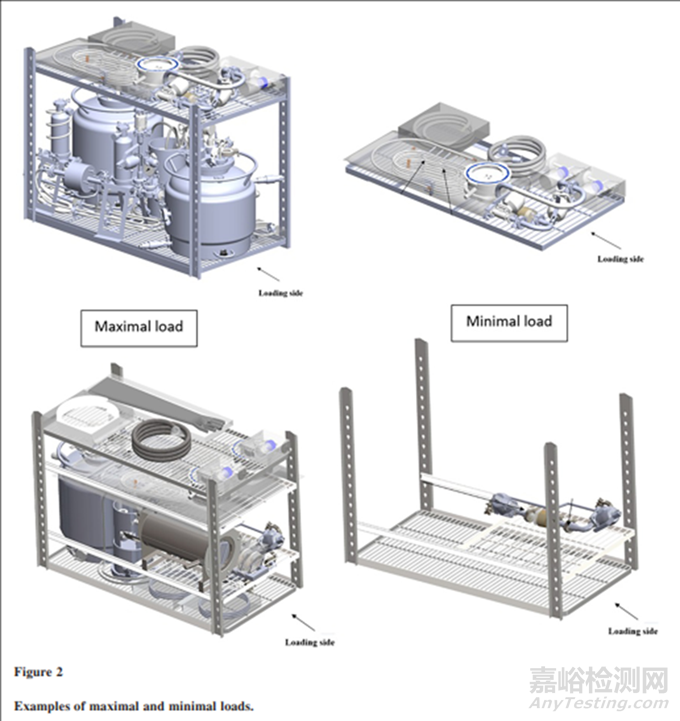
5、从收集的数据里得到以下几点看法
There are several insights from the data collection
1. Minimal load F0 values were within the values that were obtained in the maximal load (the highest and lowest F0 values were found in the maximal load cycles) (Figure 4). These data indicate that faster air removal and faster heating, which characterize the minimal cycle, do not necessarily provide the lowest lethality values.
1.最小装载量的F0值在最大装载量周期内获得的值范围内(最大和最小的F0值出现在最大装载量周期中)。这些数据表明,最小周期特征的更快的空气去除和更快的加热,并不一定提供最低的致命性值。
2. In certain cases, the differences in F0 values between the hottest and coldest points in a specific cycle might appear to be significant, but when CT values are considered, the differences are small .
2.在特定周期中,最热点和最冷点之间的F0值差异可能看起来很显著,但当考虑到CT值时,这些差异很小。
3. Because the thermocouples that are used for the PQ study are calibrated to ±0.5˚C, some differences can be related to calibration tolerances; when this factor is considered, the results indicate excellent temperature uniformity inside all of the loads.
3.由于用于PQ研究的热电偶校准精度为±0.5˚C,一些差异可能与校准容差有关;当考虑到这个因素时,结果表明所有装载内部的温度均匀性都很好
4. The correlation was low (0.26) between the equilibration time and correlated temperature differences. It would be expected that a short equilibration time indicates faster heating of the items, thus reducing temperature differences, but the low correlation does not support this relationship. Pavell and Hughes (9) also observed this finding. It would be interesting to understand the reason, scientifically. Practically speaking, the lack of correlation has no effect on the overall conclusions that are related to the overkill cycle
4.平衡时间与相关温度差异之间的相关性很低(0.26)。人们可能会预期,较短的平衡时间表明物品加热更快,从而减少温度差异,但低相关性并不支持这种关系。Pavell和Hughes(9)也观察到了这一发现。从科学上理解这一原因将是有趣的。实际上,缺乏相关性对与过度杀菌周期相关的总体结论没有影响。
The results of the data collection and analysis show clearly that the differences in lethality between loads and between items in the load are small. The large differences in the mass or density of arrangement of the items between the maximal and minimal loads did not have any effect or direction that was related to the lethality that was gained by the items.
数据分析和收集的结果清楚地表明,装载量之间以及装载量内物品之间的致命性差异很小。在最大装载量和最小装载量之间,物品的质量或排列密度的大差异并没有对物品获得的致命性产生任何相关的影响或趋势。
When evaluating the results for an overkill cycle, the differences are negligible. No item can be considered “hard to sterilize” or “challenging”, because all items achieved lethality values far beyond the initial requirements of the overkill cycle (F0 ≥ 15 min, which also covers the EU requirements) and the sterility assurance level that was required.
在评估过度杀菌周期的结果时,差异是可以忽略不计的。没有任何物品可以被视为“难以灭菌”或“具有挑战性”,因为所有物品的致命性值都远远超过了过度杀菌周期的初始要求(F0 ≥ 15分钟,这也符合欧盟的要求)以及所需的无菌保证水平。
6、结 论
Conclusions
Overkill cycles, as currently used in industry, are excessive, attaching overly conservative safeguards to safeguards. The approach of allowing flexible loads for validated items, if implemented with the principles following, will result in safe and effective sterilization cycles at far lower cost to industry and the environment.
过度杀菌周期,按照目前工业中的使用方式,是过度的,它在保护措施上增加了过于保守的防护措施。如果按照以下原则实施允许已验证物品的灵活装载的方法,将能够在大幅降低工业和环境成本的同时,实现安全有效的灭菌周期
The overkill cycle provides safe and robust sterilization for any load configuration if certain simple and basic principles are always followed:
过度杀菌周期,如果始终遵循一些简单和基本原则,可以为任何装载配置提供安全和可靠的灭菌
1. The cycle should be an extended overkill cycle. To allow load flexibility, more robust overkill cycles, with a calculated F0 value >25 minutes, are recommended to ensure the destruction of Geobacillus stearothermophilus BIs.
1.周期应为扩展的过度杀菌周期:为允许装载灵活性,推荐使用计算出的F0值大于25分钟的更强大的过度杀菌周期,以确保能够破坏嗜热脂肪芽孢杆菌(Geobacillus stearothermophilus)生物指示剂(BIs)
2. A validated item is one that has been validated by three penetration cycles (using thermocouples and BIs that are inserted into adjacent locations inside of the item) in any loading pattern. The term “item” includes the specific wrapping that is used when validated.
2.验证物品:经过验证的物品是指在任何装载模式下,通过三次穿透周期(使用插入物品内部相邻位置的热电偶和生物指示剂)进行验证的物品。“物品”一词包括在验证时使用的具体包装方式
3. The item must be placed in the same orientation in which it was validated and in a manner that will not block air or steam passage to other items.
3.物品的放置:物品必须以其验证时相同的方向放置,并且不得阻碍空气或蒸汽流向其他物品。
4. Due to greater condensation from heavy metal items, they should be placed on the lower shelves to avoid dripping onto another item
4.重物的放置:由于重金属材料会产生更多的凝结水,应将它们放置在较低的架子上,以避免水滴落到其他物品上。
5. The air removal stage should be designed to provide redundancy, such that even with fast pulses, it will be efficient. (If the autoclave is capable of controlling the timing of the pulses to be equal for maximal and minimal loads, the minimal load cycle validation is no longer relevant, and it could be skipped or be performed only once to ease regulatory questions [19]).
5.空气去除阶段的设计:应设计具有冗余性的空气去除阶段,以便即使在快速脉冲的情况下也能保持高效率。如果灭菌器能够控制脉冲时间,使最大和最小负载的脉冲时间相等,那么最小负载周期验证就不再相关了,可以省略或仅执行一次以便于监管问题的解答。
6. Revised or new loads are created under a change control procedure or a detailed SOP that includes full documentation and pictures of the revised or new load
6.新装载或修订装载的创建:新装载或修订装载应在变更控制程序或详细标准操作程序(SOP)下创建,包括完整的文档记录和修订或新装载的图片。
7、总体结论可以总结如下
The overall conclusion can be summarized as follows
If the preceding principles are followed, an item that has been validated in a specific overkill cycle will be effectively sterilized in any other load configuration using the same sterilization cycle parameters (including the air removal phase parameters), wrapping, and orientation. No additional validation is required to ensure that the combination of such items will be sterilized properly.
如果遵循前述原则,那么在一个特定的过度杀菌周期中经过验证的物品将在任何其他使用相同灭菌周期参数(包括空气去除阶段参数)、包装和方向的装载配置中被有效灭菌。为了确保这些物品的组合能够被正确灭菌,不需要额外的验证。(这意味着,一旦物品在特定的过度杀菌周期中被验证,就可以在不同的装载条件下信任其灭菌效果,而无需为每种新的装载配置重复验证过程。这为灭菌过程提供了灵活性和效率,同时保持了所需的无菌保证水平。)
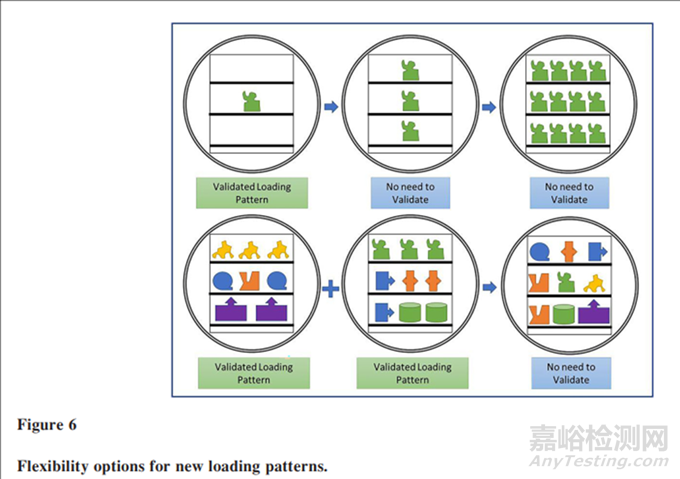
Consequently, several options are created when applying the preceding principles, such as the following:
应用前述原则后,会创建几种选择,例如:
1. A new item can be tested alone, running 3 penetration cycles, after which it can be multiply or combined with any other validated item.
1.单独测试新物品:新物品可以单独进行测试,运行3次穿透周期,之后它可以被多次使用或与其他经过验证的物品组合。
2. A combination of any validated item in any number is allowed
2.任何数量的经过验证的物品组合:允许将任何数量的经过验证的物品组合在一起。
The two options are demonstrated in Figure 6. Allowing facilities to use the preceding approach will provide flexibility in their operation and can significantly reduce the validation workload without any adverse impact on patient safety
图6展示了两种选项。允许设施采用前述方法将为其运营提供灵活性,并且可以在不牺牲患者安全的前提下显著减少验证工作量。这种方法有助于提高灭菌过程的效率,同时确保灭菌效果满足安全标准。

来源:GMP的那些事


