您当前的位置:检测资讯 > 法规标准
嘉峪检测网 2024-08-15 18:54
Clarity in GMP Guidance Pharmaceutical lsolator Leak Integrity Classes and Leak Rates
GMP指南中药品隔离器泄漏完整性等级和泄漏率的明确性
Background to development of PHSS Guidance on Barrier Isolator technology Leak integrity classes and leak rates/ acceptance criteria for qualification and routine monitoring.
PHSS关于屏障隔离技术指导文件的背景是开发泄漏完整性等级和泄漏率/验收标准,用于资格认证和常规监测。
Complex pharmaceutical isolator systems cannot comply with the requirements set out in the older generic Standard ISO 10648-2 that defines four leak integrity classes
复杂药品隔离系统无法符合旧版通用标准ISO 10648-2的要求,该标准定义了四个泄漏完整性等级
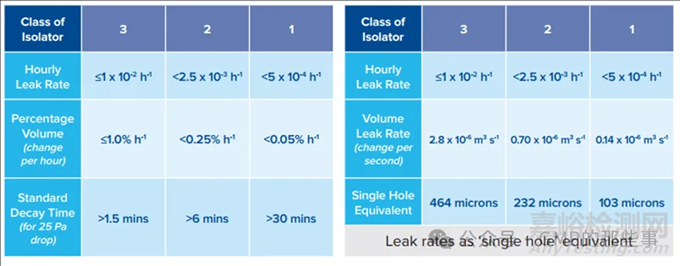
In the ISO standard the highest class 1 at 0.05% volume/hour leak rates typically applies to rigid nuclear glove boxes or inert atmosphere glove boxes used for oxygen and moisture sensitive material processing that can be tested at high pressures and over long test periods e.g. over 1000 pascal test pressures with up to one hour test periods (with result adjustments for variations of temperature and atmospheric pressure through the test period). Such high integrity, extended test periods and relatively high test pressures are not practical to apply to complex pharmaceutical isolators e.g. filling lines
在ISO标准中,最高等级1的0.05%/小时的泄漏率通常适用于刚性核手套箱或惰性气氛手套箱,这些手套箱用于对氧气和水分敏感的材料处理,可以在高压下进行测试,并在长时间测试期间(例如超过1000帕斯卡的测试压力,测试时间长达一小时,并且根据测试期间温度和大气压力的变化进行结果调整)。这种高完整性、延长的测试周期和相对较高的测试压力对于复杂的药品隔离器(例如灌装线)来说是不切实际的。
The ISO Class 2 leak integrity at 0.25% vol/h typically applies to small flexible film isolators and containment isolators and requires relatively high test pressures and extended test periods (in range of 30 minutes). Integrity testing at this level of integrity and extended test time is also not considered practical for production scale pharmaceutical isolators
ISO 2级泄漏完整性0.25%/小时通常适用于小型柔性薄膜隔离器和密闭隔离器,需要相对较高的测试压力和延长的测试周期(大约30分钟)。在这种完整性水平和延长的测试时间内进行完整性测试,对于生产规模的药品隔离器来说也不实用。
The ISO Class 3 leak integrity at 1% vol/h can applied to small scale pharmaceutical isolators in comparison to small laboratory/ containment Isolators the ISO10648- 2 standard was mainly drafted around. However as a whole the ISO standard is considered impractical to apply to the full range of Pharmaceutical isolators including larger scale filling Isolator systems that include complex sealing systems with
integrated process machinery. Such complex Filling isolators were not fully developed when this ISO standard was written hence not considered in drafting.
ISO 3级泄漏完整性1%/小时可以应用于小型药品隔离器,与ISO10648-2标准主要起草时考虑的小型实验室/密闭隔离器相比。然而,作为一个整体,ISO标准被认为不适用于包括具有复杂密封系统和集成工艺机械的大规模灌装隔离器系统在内的全部药品隔离器。这种复杂的灌装隔离器在ISO标准起草时并未完全开发,因此在起草时未被考虑。
The lowest ISO class 4 leak integrity at 10% vol/h leak rate presents at leak rate that cannot be automated via a typically applied pressure decay method (instead requires a pressure hold method that is more suitable for duct work integrity testing) and would not safely contain a gaseous bio-decontamination agent to avoid operator exposure at the OEL. Such a high level of leakage is not considered acceptable for Pharmaceutical isolators.
最低的ISO 4级泄漏完整性在10% vol/h泄漏率下,其泄漏率不能通过通常应用的压力衰减法自动化(而是需要更适合管道工作完整性测试的压力保持法),并且不能安全地包含气态生物去污剂,以避免操作人员暴露在OEL。对于药物隔离器来说,如此高的泄漏水平是不可接受的。
In a Pharmaceutical isolator barrier system for a filling line the complete system integrity combines leakage through the barrier and leakage through process machine bedplates. Typically a leak integrity of 1% vol/h is applied to the Machine ‘Bed plate’ alone that is subsequently integrated within a full barrier system.
在灌装生产线的制药隔离屏障系统中,完整的系统完整性结合了通过屏障和通过工艺机器床板的泄漏。通常,仅对机器“床板”施加1% vol/h的泄漏完整性,即随后集成在一个完整的屏障系统中。
Within the PHSS leak integrity classes that defines a range from 1% to 5% vol/h an allowance is made to accommodate bed plate leakage in the overall integrity test. PHSS leak integrity classes apply to small laboratory scale pharmaceutical isolators without bed plate integrations but including barrier penetrations e.g. Rapid transfer ports, sterility test pumps through larger complex aseptic process filling isolators
在PHSS泄漏完整性等级中,定义了1%到5%/小时的范围,允许在整体完整性测试中容纳底板泄漏。PHSS泄漏完整性等级适用于没有底板集成但包括屏障穿透的小规模实验室药品隔离器,例如快速转移端口,无菌测试泵,通过更复杂的无菌工艺灌装隔离器。
Aseptic-Containment may also be required in which case a higher leak integrity class may be specified to manage risks in containment of hazardous sterile products.
可能还需要无菌-密闭,这种情况下可能会指定更高泄漏完整性等级,以管理危险无菌产品的密闭风险。
The limit of 5% vol/h is considered as a maximum that can be applied to an integrity test using the automated pressure decay method without imposing high test pressures to allow for pressure decay without dropping below the operating pressure. Such a level of leak integrity is suitable for complex pharmaceutical isolators used for aseptic processing.
5%/小时的限制被认为是使用自动化压力衰减方法进行完整性测试的最大值,而不会施加高压以允许压力衰减而不降至操作压力以下。这种泄漏完整性水平适用于用于无菌工艺的复杂药品隔离器。
Based on Cleanroom space HVAC/ Air handling dilution of any VHP/VH202 leakage calculations can demonstrate room VHP concentrations cannot reach OEL levels of 1ppm at 5% vol/h leakage.
基于洁净室空间HVAC/空气处理对任何VHP/VH202泄漏的稀释,计算可以证明房间VHP浓度在5%/小时的泄漏下无法达到1ppm的OEL水平。
Implementing Pharmaceutical Isolator integrity testing.
实施药品隔离器完整性测试
Pharmaceutical isolator leak integrity testing is typically applied at a test pressure of a minimum of 2X the isolator operating pressure allowing for the fact that after the allowable pressure decay the final test result should not be lower than the operating pressure.
药品隔离器泄漏完整性测试通常在至少是隔离器操作压力2倍的测试压力下应用,允许在允许的压力衰减之后,最终的测试结果不应低于操作压力。
The short test periods significantly reduce the risk of false fail results from unexpected variations of temperature and barometric pressure during the test period. Leak integrity rates are based on stable test environment conditions as both temperature and atmospheric pressure fluctuations impact the pressure decay result e.g. a variation of
temperature in the tested volume of 1 degree centigrade correlates to approximately 350 pascal pressure variation and 0.07 degrees centigrade correlates to a pressure variation of approximately 25 pascal plus variation of atmospheric pressure of 1mbar
results in 100 pascal pressure variation during integrity testing. Such significant variation can take a significant portion of the acceptance criteria and can lead to a false fail result. Consideration should also be given to pressure differential change impact as operators enter cleanrooms with corresponding fluctuations as doors open and close.
短测试周期显著降低了由于测试期间意外的温度和气压变化而导致的误判风险。泄漏率基于稳定的测试环境条件,因为温度和大气压力波动都会影响压力衰减结果。例如,测试体积中温度变化1℃,大约对应于350Pa的压力变化,而0.07℃的变化对应于大约25pa的压力变化,加上大气压力1mbar的变化,结果是在完整性测试期间100帕斯卡的压力变化。这种显著的变化可能会占据验收标准的重要部分,并可能导致误判。还应考虑操作员进入洁净室时压力差变化的影响,以及门开启和关闭时的相应波动。
Application of leak integrity testing should be considered before zone disinfection and after batch production (book ending production operations) to support any root cause investigation if EM later reports a contamination event.
应该考虑在区域消毒之前应用泄漏完整性测试,并在批量生产之后(生产操作结束)进行,以支持任何根本原因调查(如果EM报告污染事件)。
It is not possible to continuously monitor Isolator leak integrity and other strategies are required to monitor impact of isolator leak integrity loss during production operations e.g. environmental monitoring and physical changes in pressure differentials. Such process monitoring will have limitations in detection of leak integrity loss during production operations so it is important to trend leak integrity test results so this CPP; critical process parameter can be assessed in performance and risk assessed for impact as a result of deviation.
无法连续监测隔离器泄漏完整性,需要其他策略来监测生产操作期间隔离器泄漏完整性损失的影响,例如环境监测和压差的身体变化。这种过程监测在检测生产操作期间泄漏完整性损失方面将有局限性,因此重要的是要趋势化泄漏完整性测试结果,以便这种CPP;关键过程参数可以在性能上进行评估,并针对偏差的影响进行风险评估
If negative pressure operation would be applied it follows to reduce the leak hole sizes in an isolation system to a level below which a cfu could not pass through (0.2 micron), the isolator barrier would need to act like a HEPA filter with high levels of leak integrity which are impractical for construction of pharmaceutical isolators used for aseptic
processing.
如果应用负压操作,则需要将隔离系统的泄漏孔径减小到cfu无法通过的水平(0.2微米),隔离器屏障需要像HEPA过滤器一样具有高水平的泄漏完整性,这对于用于无菌处理的药品隔离器的构建是不切实际的。
Qualification and routine monitoring of Pharmaceutical Isolator barrier leak integrity.
药品隔离器屏障泄漏完整性的确认和常规监测
For reference; Classification according ISO 10648-2 – Generic standard for Containment Isolators.
参考;根据ISO 10648-2分类 - 密闭隔离器的通用标准。

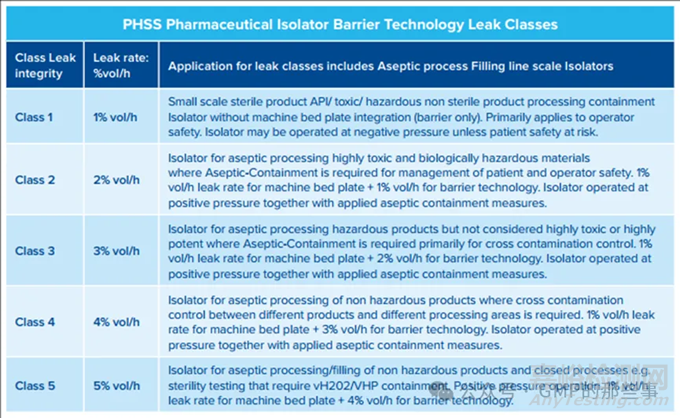
PHSS Classification classes for Pharmaceutical Isolators including larger complex Aseptic processing Filling Isolators.
PHSS为药品隔离器定义了五个隔离器屏障泄漏完整性等级,包括更复杂的无菌工艺灌装隔离器。
The PHSS defines five classes of Isolator barrier leak integrity with each class corresponding to the relative %volume per hour leak rate:
PHSS定义了五种隔离器屏障泄漏完整性等级,每个等级对应于相对的每小时体积百分比泄漏率:
• 1% vol/h = PHSS Class 1
• 2% vol/h = PHSS Class 2
• 3% vol/h = PHSS Class 3
• 4% vol/h = PHSS Class 4
• 5% vol/h = PHSS Class 5
Therefore PHSS Class 1 leak integrity would correspond to the ISO 10648-2 Class 3 leak integrity.PHSS leak integrity classes are specific to Pharmaceutical isolators and not generic to all industries.
因此,PHSS 1级泄漏完整性将对应于ISO 10648-2的3级泄漏完整性。PHSS泄漏完整性等级特定于药品隔离器,并不适用于所有行业。
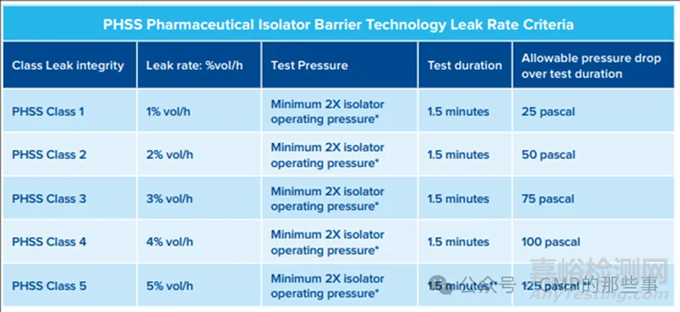
Test pressures* a minimum of 2X the operating Isolator pressure should be applied as a test pressure with the minimum value also taking into account that following the allowable pressure decay the final test pressure should not be lower that the isolator operating pressure. High test pressures should be avoided to prevent unnecessary stress placed on sealing systems and the structure of the barrier system
测试压力* 应将操作隔离器压力的至少2倍作为测试压力应用,最低值还应考虑在允许的压力衰减之后,最终测试压力不应低于隔离器操作压力。应避免高测试压力,以防止对密封系统和屏障结构造成不必要的压力。
Test duration** the test durations should be kept short to avoid potential impact of temperature and barometric pressure fluctuations. In the case of the test pressure for integrity testing at the 5% vol/h leak integrity class the test duration could be reduced to 1 minute with an allowable pressure drop of 100 pascal if the test pressure is considered to be excessive in terms of applied stress to the isolator barrier when the 1.5 minute test duration is applied. There does need to be enough allowable margin from the test pressure to assure after pressure decay from leak testing the resulting pressure is no less than the intended operating pressure of the isolator.
测试持续时间** 测试持续时间应保持短暂,以避免温度和气压波动的潜在影响。在5%/小时泄漏完整性等级的完整性测试中,如果认为1.5分钟的测试持续时间对隔离器屏障施加的压力过大,测试持续时间可以减少到1分钟,允许的压力下降为100帕斯卡。测试压力需要有足够的允许余量,以确保泄漏测试后的压力衰减结果不小于隔离器的预定操作压力。
Positive operating pressure pharmaceutical isolators should be pressure decay leak tested at a positive test pressure at a minimum of 2X the normal isolator operating pressure.
正压操作的药品隔离器应在正常隔离器操作压力的至少2倍的正压测试压力下进行压力衰减泄漏测试。
Negative pressure operation isolators must be qualified by pressure decay leak tests at a negative test pressure. In addition it may be useful to initially test, prior to commissioning, via an alternative test method to apply a positive test pressure so test agent e.g. ammonia, helium out leakage can be detected to assist diffused leakage assessments.
负压操作隔离器必须在负压测试压力下通过压力衰减泄漏试验。此外,在调试之前,通过另一种测试方法(施加正测试压力)进行初始测试可能是有用的,这样可以检测到测试剂(如氨、氦)的泄漏,以辅助扩散泄漏评估。
Integrated process machine integrity: By default integrity of machine bed plates applies as 1% vol/h (25 pascal allowable pressure drop over 1.5 minute test duration). At factory qualification testing (FQT and FAT) where filling machines may not be installed with the isolator and testing is completed with a dummy blank bed plate acceptance criteria for the Isolator barrier should be applied less 1% vol/h allowance for the machine bed plate sealing integrity that would form the complete barrier boundary later in Operational Qualification (OQ) studies
集成工艺设备的完整性:默认情况下,机器底板的完整性适用于1%/小时(1.5分钟测试持续时间内允许的压力下降为25帕斯卡)。在工厂资格测试(FQT和FAT)中,如果灌装机器没有与隔离器一起安装,并且测试是使用虚拟空白底板完成的,则隔离器屏障的验收标准应减去1%/小时的机器底板密封完整性,这将在操作资格(OQ)研究中形成完整的屏障边界。
Qualification and routine monitoring leak integrity testing.
确认和常规监测泄漏完整性测试。
The values of test criteria in the PHSS leak integrity class tables are considered appropriate for qualification and routine monitoring however in principle a qualification test could pass with just a few pascal margin so considerations are required to assure robust margins in subsequent routine leak integrity monitoring. It is recommended either to consider more test pass margin at FQT/FAT testing to allow for inherent fluctuation in process operations (to allow for robust performance) or apply an extra margin of allowable pressure drop for routine monitoring to provide a more robust operating margin
PHSS泄漏完整性等级表中的测试标准值被认为适用于确认和常规监测,但原则上,确认测试可能仅以几帕斯卡的余量通过,因此需要考虑确保在随后的常规泄漏完整性监测中具有强大的余量。建议在FQT/FAT测试中考虑更多的测试通过余量,以允许工艺操作中的固有波动(以实现强大的性能),或在常规监测中应用额外的允许压力下降余量,以提供更强的操作余量。
It is considered an extra 10 pascal of allowable pressure drop is justifiable in routine monitoring considering pressure monitoring device accuracy and small variations from reference devices during routine calibrations: Reference: EJPPS publication 2102; 17(3) 120- 125: An improved leak integrity rationale for Pharmaceutical isolators: Drinkwater, Triggs. Although this publication considers only small scale pharmaceutical/ pharmacy isolators the principles of the rationale are valid for larger scale isolators including filling lines.
额外的10帕斯卡的允许压力下降在常规监测中被认为是合理的,考虑到压力监测设备在常规校准期间的准确性和与参考设备的小变化:参考:EJPPS出版物2102;17(3)120-125:改进的药品隔离器泄漏完整性理由:Drinkwater,Triggs。尽管这个出版物只考虑了小规模的药品/药房隔离器,但其理由的原则对于包括灌装线在内的更大规模的隔离器是有效的。
In all cases a stabilization time should be applied before qualification/ routine pressure decay testing commences to allow for physical stabilization e.g. glove material stretching, barrier wall flexing as result of the applied test pressure. Stabilization times, typically a minimum of 1 minute, should be characterized for isolator systems where pressure decay testing is applied.
在所有情况下,在开始确认/常规压力衰减测试之前,应该应用稳定时间,以允许物理稳定,例如手套材料拉伸,由于应用的测试压力而使屏障壁弯曲。对于应用压力衰减测试的隔离器系统,稳定时间通常至少为1分钟。
During routine leak integrity testing if there is a marginal failure of integrity it is recommended to define (by procedure) a repeat leak test to account for potential temperature changes and a false fail result. If there is a rapid drop in pressure during testing with a slowing of pressure drop as the acceptance criteria is reached this often indicates a temperature impact (cooling of pressurised test air). Actual leaks tend to drop pressure more immediately and consistently.
在常规泄漏完整性测试中,如果存在边缘完整性失败,建议(通过程序)定义重复泄漏测试,以解释潜在的温度变化和错误的失败结果。如果在测试过程中压力迅速下降,并且达到验收标准后压力下降速度减慢,这通常表明温度影响(加压测试空气冷却)。实际泄漏往往会更迅速、更持续地降低压力。
Leak integrity test automation and records.
泄漏完整性测试自动化和记录
Automated pressure decay systems should ideally be integrated into the control system of the isolator barrier with test records including pass and fail results that meet data integrity requirements, included within the Isolator Batch / operating records. There should be risk based rationale to define the isolator leak integrity test parameters and associated acceptance criteria for qualification and routine integrity monitoring with details documented in the CCS (Contamination Control Strategy)
理想情况下,自动化压力衰减系统应集成到隔离器屏障的控制系统中,测试记录包括符合数据完整性要求的合格和不合格结果,包括在隔离器批次/操作记录中。应该有基于风险的基本原理来定义隔离器泄漏完整性测试参数和相关的验收标准,以确认和例行完整性监测,详细信息记录在CCS(污染控制策略)中。
Automation of the leak integrity test should include the automated closure of barrier dampers that form the controlled zone/ containment zone, management of integrated EM sampling systems (typically placing sample lines into a recirculation loop via a divert valve ahead of the particle counter and/or active air sampler) and closing of any isolator automated ‘Mouse holes’. In addition for isolators subjected to VHP/VH202 Bio-decontamination gloves need to be positioned on glove extenders (that are used for glove VHP exposure) so they are presented in a controlled manner for integrity testing. Gloves that hang loosely out of the isolator integrity during testing may be subjected to movement (dropping) during pressure decay providing a volume change that by default compensates the pressure drop and can change the test result to a more favourable but false outcome.
泄漏完整性测试的自动化应包括形成控制区/安全区的屏障阻尼器的自动关闭,集成EM采样系统的管理(通常通过颗粒计数器和/或主动空气采样器前面的分流阀将样品线放入再循环回路中)以及关闭任何隔离器的自动“老鼠洞”。此外,对于受VHP/VH202影响的隔离器,生物去污手套需要放置在手套扩展器上(用于手套VHP暴露),以便以受控的方式呈现,以进行完整性测试。在测试过程中,松散地悬挂在隔离器完整性外的手套可能会在压力衰减期间受到移动(掉落)的影响,从而提供默认情况下补偿压降的体积变化,并可能将测试结果更改为更有利但错误的结果。
Isolator integrity testing should be completed before implementing a VHP/VH202 cycle to ensure the isolator leak integrity CPP meets that defined at which Grade A conditions are established and the isolator is safe to contain VHP/VH202 once the bio-decontamination cycle is started. As a critical process parameter it is recommended the pre-production leak integrity test is applied as a control system ‘interlock’ so the control sequence will not advance to the VHP/VH202 cycle and on to production operations if the integrity test fails
隔离器完整性测试应在实施VHP/VH202循环之前完成,以确保隔离器泄漏完整性CPP符合建立a级条件的定义,并且一旦生物净化循环开始,隔离器可以安全容纳VHP/VH202。作为一个关键的工艺参数,建议将生产前泄漏完整性测试作为控制系统的“联锁”应用,这样,如果完整性测试失败,控制序列就不会进入VHP/VH202循环,也不会进入生产操作。
A post production/ batch isolator leak integrity test is recommended to confirm there was a steady state of leak integrity through production operations and support any route cause investigation as a result of a contamination event. After the automated pre-production Isolator leak integrity ‘interlock’ test further integrity tests should be selectable with rationale defined for the frequency
建议在生产/批次后进行隔离器泄漏完整性测试,以确认生产操作期间泄漏完整性的稳定状态,并支持由于污染事件而进行的任何根本原因调查。在自动化生产前隔离器泄漏完整性“互锁”测试之后,应可选择进一步的完整性测试,并为频率定义理由。
Barrier glove leak integrity is subject of separate PHSS guidance and should be undertaken separately to isolator barrier leak integrity testing (covered by this guidance) as the leak detection sensitivity for the full isolator volume is insufficient to detect single pin holes in gloves at around 100 micron diameter (the typical limit of detection for glove integrity test devices). Studies demonstrated it took 20 needle stick punctures of a barrier glove to fail an isolator leak integrity test at the PHSS Class 1 (1% vol/h) and ISO 10648-2 Class 3 (1% vol/h) leak test level.
屏障手套泄漏完整性是单独的PHSS指导的主题,应与本指南涵盖的隔离器屏障泄漏完整性测试分开进行,因为对于整个隔离器体积的泄漏检测灵敏度不足以检测手套中约100微米直径的单个针孔(手套完整性测试设备的典型检测限制)。研究表明,需要20次针刺穿透屏障手套,才能在PHSS 1级(1%/小时)和ISO 10648-2 3级(1%/小时)泄漏测试水平下使隔离器泄漏完整性测试失败。

来源:GMP的那些事


