您当前的位置:检测资讯 > 实验管理
嘉峪检测网 2024-11-07 08:57
乙腈特性
Acetonitrile characteristics
乙腈,常温常压下为无色透明液体,极易挥发,有优良的溶剂性能,能溶解多种有机、无机和气体物质,与水和醇无限互溶,有类似于醚的特殊气味。
Acetonitrile is a colorless and transparent liquid under normal temperature and pressure. It is highly volatile and has excellent solvent properties. It can dissolve a variety of organic, inorganic and gaseous substances. It is infinitely miscible with water and alcohol and has a special smell similar to ether.
乙腈虽然包“溶”性强,但是也是有脾气的,很危险。乙腈为有毒品,具有燃烧、爆炸性质,遇明火、高热能引起燃烧爆炸,因其蒸气比空气重,能在较低处扩散到相当远的地方,遇火源引着回燃。同时,乙腈可经呼吸道和皮肤黏膜被吸收进人体,其致死量为5mL,所以使用人员需做好防护。
Although acetonitrile has strong "soluble" properties, it is also temperamental and very dangerous. Acetonitrile is a poisonous substance with flammable and explosive properties. It can cause combustion and explosion when exposed to open flames or high heat. Because its vapor is heavier than air, it can spread to a considerable distance at a lower level and cause backfire when encountering a fire source. At the same time, acetonitrile can be absorbed into the human body through the respiratory tract and skin and mucous membranes , with a lethal dose of 5 mL, so users need to take precautions.
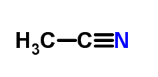
乙腈是一种极性的不可电离非质子酸,由于存在由于存在C≡N,它可以带来π-π相互作用,又因为氮原子具有高电负性,它可以作为氢键的受体与另一分子形成氢键。
Acetonitrile is a polar non-ionizable aprotic acid. Due to the presence of C≡N, it can bring about π-π interaction, and because the nitrogen atom has high electronegativity, it can serve as a hydrogen bond acceptor with Another molecule forms a hydrogen bond.
甲醇特性
Methanol characteristics
甲醇,是一种无色透明、易燃、有毒的高挥发液体。结构为最简单的饱和一元醇,纯品略带乙醇气味,粗品刺鼻难闻,能无限地溶于水或酒精中。甲醇最早从干馏木材的蒸出液中分离得到,故又称“木醇”或“木酒精”。
Methanol is a colorless, transparent, flammable, toxic and highly volatile liquid. The structure is the simplest saturated monohydric alcohol. The pure product has a slight ethanol smell, while the crude product is pungent and unpleasant. It can be infinitely soluble in water or alcohol. Methanol was first separated from the distillate of dry distillation wood, so it is also called "wood alcohol" or "wood alcohol".
甲醇有毒,中毒的潜伏期一般介于12-24小时。致死剂量为30ml,5~10ml为严重中毒,10ml以上可引起失明,可经人体皮肤、呼吸道、胃肠道吸收中毒,所以操作使用时需注意。
Methanol is toxic, and the incubation period for poisoning is generally between 12 and 24 hours. The lethal dose is 30ml, 5 to 10ml is severe poisoning, and more than 10ml can cause blindness. It can be absorbed through human skin, respiratory tract, and gastrointestinal tract, so please pay attention when handling it.
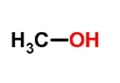
甲醇是一种极性的可电离质子酸,它可以和另一个分子相互作用形成氢键。
Methanol is a polar, ionizable protic acid that can interact with another molecule to form hydrogen bonds.
Q:为什么甲醇可以形成氢键呢? Why can methanol form hydrogen bonds?
A:因为甲醇上有-OH的存在,氧是高电负性原子,它可以部分极化分子。它在氧上产生微负电荷,在氢原子上产生微正电荷。因此,这个微正电荷的氢原子可以与微负电荷的原子形成氢键。
Because of the presence of -OH on methanol, oxygen is a highly electronegative atom, which can partially polarize the molecule. It creates a slightly negative charge on oxygen and a slightly positive charge on hydrogen atoms. Therefore, this slightly positively charged hydrogen atom can form a hydrogen bond with a slightly negatively charged atom.
Q:什么是极性可电离质子溶剂? What are polar ionizable protic solvents?
A:极性可电离质子溶剂指的是具有氢原子与氧(如羟基)、氮(如氨基)或氟(如氟化氢)结合的溶剂。乙腈中存在氢原子,但这些氢原子不与氧形成氢键,因此,不能说乙腈是可电离的极性溶剂。而甲醇,氢原子可以与氧形成氢键,因此,甲醇是可电离极性溶剂。
Polar ionizable protic solvents are solvents that have hydrogen atoms bonded to oxygen (such as hydroxyl groups), nitrogen (such as amino groups), or fluorine (such as hydrogen fluoride). There are hydrogen atoms in acetonitrile, but these hydrogen atoms do not form hydrogen bonds with oxygen. Therefore, acetonitrile cannot be said to be an ionizable polar solvent. In methanol, hydrogen atoms can form hydrogen bonds with oxygen, so methanol is an ionizable polar solvent.

来源:Internet


