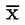COMMISSION REGULATION (EU) 2017/644 of 5 April 2017
laying down methods of sampling and analysis for the control of levels of dioxins, dioxin-like PCBs and non-dioxin-like PCBs in certain foodstuffs and repealing Regulation (EU) No 589/2014
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EC) No 882/2004 of the European Parliament and of the Council of 29 April 2004 on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare rules (1), and in particular Article 11(4) thereof,
Whereas:
|
(1) |
Commission Regulation (EC) No 1881/2006 (2) sets out the maximum levels for non-dioxin-like polychlorinated biphenyls (PCBs) dioxins and furans and for the sum of dioxins, furans and dioxin-like PCBs in certain foodstuffs. |
|
(2) |
Commission Recommendation 2013/711/EU (3) sets out action levels in order to stimulate a proactive approach to reduce the presence of polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans (PCDD/Fs) and dioxin-like PCBs in food. Those action levels are a tool used by competent authorities and operators to highlight those cases where it is appropriate to identify a source of contamination and to take the necessary measures in order to reduce or eliminate it. |
|
(3) |
Commission Regulation (EC) No 589/2014 (4) establishes specific provisions concerning the sampling procedure and the methods of analysis to be applied for the official control of levels of dioxins, dioxin-like PCBs and non-dioxin-like PCBs. |
|
(4) |
The provisions laid down in this Regulation relate only to the sampling and analysis of dioxins, dioxin-like PCBs and non-dioxin-like PCBs for the implementation of Regulation (EC) No 1881/2006 and Recommendation 2013/711/EU. They do not affect the sampling strategy, sampling levels and frequency as set out in Annexes III and IV to Council Directive 96/23/EC (5). They do not affect the targeting criteria for sampling as laid down in Commission Decision 98/179/EC (6). |
|
(5) |
It is appropriate to ensure that food business operators applying the controls performed within the framework of Article 4 of Regulation (EC) No 852/2004 of the European Parliament and of the Council (7) apply sampling procedures equivalent to the sampling procedures provided for by this Regulation in order to ensure that samples taken for those controls are representative samples. Furthermore, the European Union Reference Laboratory for Dioxins and PCBs has provided evidence that analytical results in certain cases are not reliable when the performance criteria as provided in this Regulation are not applied by laboratories performing the analysis of samples taken by food business operators within the framework of Article 4 of Regulation (EC) No 852/2004. It is therefore appropriate to make the application of the performance criteria also obligatory for the analysis of those samples. |
|
(6) |
Given that the approach of the use of a decision limit to ensure that an analytical result is above the maximum level with a certain probability, as provided for in Commission Decision 2002/657/EC (8), is no longer applied for the analysis of dioxins and PCBs in food, it is appropriate to delete this approach and to keep only the approach of the expanded uncertainty using the coverage factor of 2, giving a level confidence of approximately 95 %. |
|
(7) |
In line with the reporting requirements for bioanalytical screening methods, it is appropriate to also provide for physico-chemical methods used for screening specific reporting requirements. |
|
(8) |
Given that the analysis of dioxins, dioxin-like PCBs and non-dioxin-like PCBs are in most cases determined together it is appropriate to align the performance criteria for the non-dioxin-like PCBs to the performance criteria for dioxins and dioxin-like PCBs. This is a simplification, without substantial changes in practice as in the case of non-dioxin-like PCBs the relative intensity of qualifier ions compared to target ions is > 50 %. |
|
(9) |
Furthermore there are several other minor modifications proposed to the current provisions, requiring the repeal of Regulation (EU) No 589/2014 and its replacing by a new Regulation to maintain the readability of the text. |
|
(10) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on Plants, Animals, Food and Feed, |
HAS ADOPTED THIS REGULATION:
Article 1
For the purposes of this Regulation, the definitions and abbreviations set out in Annex I shall apply.
Article 2
Sampling for the official control of the levels of dioxins, furans, dioxin-like PCBs and non-dioxin-like PCBs in foodstuffs listed in Section 5 of the Annex to Regulation (EC) No 1881/2006 shall be carried out in accordance with the methods set out in Annex II to this Regulation.
Article 3
Sample preparation and analyses for the control of the levels of dioxins, furans and dioxin-like PCBs in foodstuffs listed in Section 5 of the Annex to Regulation (EC) No 1881/2006 shall be carried out in accordance with the methods set out in Annex III to this Regulation.
Article 4
Analyses for the control of the levels of non-dioxin-like PCBs in foodstuffs listed in Section 5 of the Annex to Regulation (EC) No 1881/2006 shall be carried out in accordance with the requirements for analytical procedures set out in Annex IV to this Regulation.
Article 5
Regulation (EU) No 589/2014 is repealed.
References to the repealed Regulation shall be construed as references to this Regulation.
Article 6
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 5 April 2017.
For the Commission
The President
Jean-Claude JUNCKER
(1) OJ L 165, 30.4.2004, p. 1.
(2) Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (OJ L 364, 20.12.2006, p. 5).
(3) Commission Recommendation 2013/711/EU of 3 December 2013 on the reduction of the presence of dioxins, furans and PCBs in feed and food (OJ L 323, 4.12.2013, p. 37).
(4) Commission Regulation (EU) No 589/2014 of 2 June 2014 laying down methods of sampling and analysis for the official control of levels of dioxins, dioxin-like PCBs and non-dioxin-like PCBs in certain foodstuffs and repealing Regulation (EU) No 252/2012 (OJ L 164, 3.6.2014, p. 18).
(5) Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC (OJ L 125, 23.5.1996, p. 10).
(6) Commission Decision 98/179/EC of 23 February 1998 laying down detailed rules on official sampling for the monitoring of certain substances and residues thereof in live animals and animal products (OJ L 65, 5.3.1998, p. 31).
(7) Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs (OJ L 139, 30.4.2004, p. 1).
(8) Commission Decision 2002/657/EC of 14 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (OJ L 221, 17.8.2002, p. 8).
ANNEX I
DEFINITIONS AND ABBREVIATIONS
I. DEFINITIONS
For the purposes of this Regulation the definitions laid down in Annex I to Decision 2002/657/EC shall apply.
Further to those definitions, the following definitions shall apply for the purposes of this Regulation:
|
BEQ |
Bioanalytical Equivalents |
|
GC |
Gas chromatography |
|
HRMS |
High resolution mass spectrometry |
|
LRMS |
Low resolution mass spectrometry |
|
MS/MS |
Tandem mass spectrometry |
|
PCB |
Polychlorinated biphenyl |
|
Non-dioxin-like PCBs |
PCB 28, PCB 52, PCB 101, PCB 138, PCB 153 and PCB 180 |
|
PCDD |
Polychlorinated dibenzo-p-dioxins |
|
PCDF |
Polychlorinated dibenzofurans |
|
QC |
Quality control |
|
REP |
Relative potency |
|
TEF |
Toxic Equivalency Factor |
|
TEQ |
Toxic Equivalents |
|
TCDD |
2,3,7,8-Tetrachlorodibenzo-p-dioxin |
|
U |
Expanded measurement uncertainty |
(1) Bioanalytical methods are not specific to those congeners included in the TEF-scheme. Other structurally related AhR-active compounds may be present in the sample extract which contribute to the overall response. Therefore, bioanalytical results cannot be an estimate but rather an indication of the TEQ level in the sample.
(2) The principles as described in the 'Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food' [link to website] shall be followed when applicable.
(3) The LOQ is calculated from the lowest concentration point taking into account the recovery of internal standards and sample intake.
ANNEX II
METHODS OF SAMPLING FOR OFFICIAL CONTROL OF LEVELS OF DIOXINS (PCDD/PCDF), DIOXIN-LIKE PCBs AND NON-DIOXIN-LIKE PCBs IN CERTAIN FOODSTUFFS
I. SCOPE
Samples intended for the official control of the levels of dioxins (PCDD/Fs), dioxin-like PCBs and non-dioxin-like PCBs in foodstuffs shall be taken according to the methods described in this Annex. Aggregate samples thus obtained shall be considered as representative of the lots or sublots from which they are taken. Compliance with maximum levels laid down in Regulation (EC) No 1881/2006 shall be established on the basis of the levels determined in the laboratory samples.
To ensure compliance with the provisions in Article 4 of Regulation (EC) No 852/2004, food business operator shall, when samples are taken to control the levels of dioxins (PCDD/Fs), dioxin-like PCBs and non-dioxin-like PCBs, take the samples according to the methods described in Chapter III of this Annex or apply an equivalent sampling procedure which is demonstrated to have a same level of representation as the sampling procedure described in Chapter III of this Annex.
II. GENERAL PROVISIONS
1. Personnel
Offcial sampling shall be performed by an authorised person as designated by the Member State.
2. Material to be sampled
Each lot or sublot which is to be examined shall be sampled separately.
3. Precautions to be taken
In the course of sampling and the preparation of the samples, precautions shall be taken to avoid any changes which would affect the content of dioxins and PCBs, adversely affect the analytical determination or make the aggregate samples unrepresentative.
4. Incremental samples
As far as possible, incremental samples shall be taken at various places distributed throughout the lot or sublot. Departure from such a procedure shall be recorded in the record provided for under point II.8.
5. Preparation of the aggregate sample
The aggregate sample shall be made up by combining the incremental samples. It shall be at least 1 kg unless not practical, e.g. when a single package has been sampled or when the product has a very high commercial value.
6. Replicate samples
The replicate samples for enforcement, defence and reference purposes shall be taken from the homogenised aggregate sample, unless such procedure conflicts with a Member State's rules as regard the rights of the food business operator. The size of the laboratory samples for enforcement shall be sufficient to allow at least for duplicate analyses.
7. Packaging and transmission of samples
Each sample shall be placed in a clean, inert container offering adequate protection from contamination, from loss of analytes by adsorption to the internal wall of the container and against damage in transit. All necessary precautions shall be taken to avoid any change in composition of the sample which might arise during transportation or storage.
8. Sealing and labelling of samples
Each sample taken for official use shall be sealed at the place of sampling and identified in accordance with the rules of the Member States.
A record shall be kept of each sampling, permitting each lot to be identified unambiguously and giving the date and place of sampling together with any additional information likely to be of assistance to the analyst.
III. SAMPLING PLAN
The sampling method applied shall ensure that the aggregate sample is representative of the (sub)lot that is to be controlled.
1. Division of lots into sublots
Large lots shall be divided into sublots on condition that the sublot can be separated physically. For products traded in large bulk consignments (e.g. vegetable oils) Table 1 shall apply. For other products Table 2 shall apply. Taking into account that the weight of the lot is not always an exact multiple of the weight of the sublots, the weight of the sublot may exceed the mentioned weight by a maximum of 20 %.
Table 1
Subdivision of lots into sublots for products traded in bulk consignments
|
Lot weight (ton) |
Weight or number of sublots |
|
≥ 1 500 |
500 tonnes |
|
> 300 and < 1 500 |
3 sublots |
|
≥ 50 and ≤ 300 |
100 tonnes |
|
< 50 |
— |
Table 2
Subdivision of lots into sublots for other products
|
Lot weight (ton) |
Weight or number of sublots |
|
≥ 15 |
15-30 tonnes |
|
< 15 |
— |
2. Number of incremental samples
The aggregate sample uniting all incremental samples shall be at least 1 kg (see point II.5).
The minimum number of incremental samples to be taken from the lot or sublot shall be as given in Tables 3 and 4.
In the case of bulk liquid products, the lot or sublot shall be thoroughly mixed insofar as possible and insofar as it does not affect the quality of the product by either manual or mechanical means immediately prior to sampling. In that case, a homogeneous distribution of contaminants is assumed within a given lot or sublot. It is therefore sufficient to take three incremental samples from a lot or sublot to form the aggregate sample.
The incremental samples shall be of similar weight. The weight of an incremental sample shall be at least 100 grams.
Departure from this procedure must be recorded in the record provided for under point II.8 of this Annex. In accordance with the provisions of Commission Decision 97/747/EC (1), the aggregate sample size for hens' eggs is at least 12 eggs (for bulk lots as well as for lots consisting of individual packages, Tables 3 and 4 shall apply).
Table 3
Minimum number of incremental samples to be taken from the lot or sublot
|
Weight or volume of lot/sublot (in kg or litre) |
Minimum number of incremental samples to be taken |
|
< 50 |
3 |
|
50 to 500 |
5 |
|
> 500 |
10 |
If the lot or sublot consists of individual packages or units, then the number of packages or units which shall be taken to form the aggregate sample is given in Table 4.
Table 4
Number of packages or units (incremental samples) which shall be taken to form the aggregate sample if the lot or sublot consists of individual packages or units
|
Number of packages or units in the lot/sublot |
Number of packages or units to be taken |
|
1 to 25 |
at least 1 package or unit |
|
26 to 100 |
about 5 %, at least 2 packages or units |
|
> 100 |
about 5 %, at maximum 10 packages or units |
3. Specific provisions for the sampling of lots containing whole fishes of comparable size and weight
Fishes are considered to be of comparable size and weight where the difference in size and weight does not exceed about 50 %.
The number of incremental samples to be taken from the lot are defined in Table 3. The aggregate sample uniting all incremental samples shall be at least 1 kg (see point II.5).
|
— |
Where the lot to be sampled contains small fishes (individual fishes weighing < about 1 kg), the whole fish is taken as incremental sample to form the aggregate sample. Where the resulting aggregate sample weighs more than 3 kg, the incremental samples may consist of the middle part, weighing each at least 100 grams, of the fishes forming the aggregate sample. The whole part to which the maximum level is applicable is used for homogenisation of the sample. The middle part of the fish is where the centre of gravity is. This is located in most cases at the dorsal fin (in case the fish has a dorsal fin) or halfway between the gill opening and the anus. |
|
— |
Where the lot to be sampled contains larger fishes (individual fishes weighing more than about 1 kg), the incremental sample consists of the middle part of the fish. Each incremental sample weighs at least 100 grams. For fishes of intermediate size (about 1-6 kg) the incremental sample is taken as a slice of the fish from backbone to belly in the middle part of the fish. For very large fishes (e.g. > about 6 kg), the incremental part is taken from the right side (frontal view) dorso-lateral muscle meat in the middle part of the fish. Where the taking of such a piece of the middle part of the fish would result in significant economic damage, the taking of three incremental samples of at least 350 grams each may be considered as being sufficient independent of the size of the lot or alternatively an equal part of the muscled meat close to the tail part and the muscle meat close to the head part of one fish may be taken to form the incremental sample being representative for the level of dioxins in the whole fish. |
4. Sampling of lots of fish containing whole fishes of different size and/or weight
|
— |
The provisions of point III.3 as regards sample constitution shall apply. |
|
— |
Where a size or weight class/category is predominant (about 80 % or more of the lot), the sample is taken from fishes with the predominant size or weight. This sample is to be considered as being representative for the whole lot. |
|
— |
Where no particular size or weight class/category predominates, then it must be ensured that the fishes selected for the sample are representative for the lot. Specific guidance for such cases is provided in ‘Guidance document on sampling of whole fishes of different size and/or weight’ (2). |
5. Sampling at retail stage
Sampling of foodstuffs at the retail stage shall be done where possible in accordance with the sampling provisions set out in point III.2.
Where this is not possible, an alternative method of sampling at retail stage may be used provided that it ensures sufficient representativeness for the sampled lot or sublot.
IV. COMPLIANCE OF THE LOT WITH SPECIFICATION
1. As regards non-dioxin-like PCBs
The lot is compliant if the analytical result for the sum of non-dioxin-like PCBs does not exceed the respective maximum level, as laid down in Regulation (EC) No 1881/2006 taking into account the expanded measurement uncertainty (3).
The lot is non-compliant with the maximum level as laid down in Regulation (EC) No 1881/2006 if the mean of two upperbound analytical results obtained from duplicate analysis (4), taking into account the expanded measurement uncertainty, exceeds the maximum level beyond reasonable doubt.
The expanded measurement uncertainty is calculated using a coverage factor of 2 which gives a level of confidence of approximately 95 %. A lot is non-compliant if the mean of the measured values minus the expanded uncertainty of the mean is above the established maximum level.
The rules, mentioned in the paragraphs above under this point, shall apply for the analytical result obtained on the sample for official control. In case of analysis for defence or reference purposes, the national rules apply.
2. As regards dioxins (PCDD/Fs) and dioxin-like PCBs
The lot is compliant if the result of a single analysis
|
— |
performed by a screening method with a false-compliant rate below 5 % indicates that the level does not exceed the respective maximum level of PCDD/Fs and the sum of PCDD/Fs and dioxin-like PCBs as laid down in Regulation (EC) No 1881/2006, |
|
— |
performed by a confirmatory method does not exceed the respective maximum level of PCDD/Fs and the sum of PCDD/Fs and dioxin-like PCBs as laid down in Regulation (EC) No 1881/2006 taking into account the expanded measurement uncertainty (5). |
For screening assays a cut-off value shall be established for the decision on the compliance with the respective maximum levels set for either PCDD/Fs or for the sum of PCDD/Fs and dioxin-like PCBs.
The lot is non-compliant with the maximum level as laid down in Regulation (EC) No 1881/2006 if the mean of two upperbound analytical results (duplicate analysis (6)) obtained using a confirmatory method, taking into account the expanded measurement uncertainty, exceeds the maximum level beyond reasonable doubt.
The expanded measurement uncertainty is calculated using a coverage factor of 2 which gives a level of confidence of approximately 95 %. A lot is non-compliant if the mean of the measured values minus the expanded uncertainty of the mean is above the established maximum level.
The sum of the estimated expanded uncertainties of the separate analytical results of PCDD/Fs and dioxin-like PCBs has to be used for the estimated expanded uncertainty of the sum of PCDD/Fs and dioxin-like PCBs,
The rules, mentioned in the paragraphs above under this point, shall apply for the analytical result obtained on the sample for official control. In case of analysis for defence or reference purposes, the national rules apply.
V. EXCEEDANCE OF ACTION LEVELS
Action levels serve as a tool for the selection of samples in those cases where it is appropriate to identify a source of contamination and to take measures for its reduction or elimination. Screening methods shall establish the appropriate cut-off values for selection of those samples. Where significant efforts are necessary to identify a source and to reduce or eliminate the contamination, it might be appropriate to confirm exceedance of the action level by duplicate analysis using a confirmatory method and taking into account the expanded measurement uncertainty (7).
(1) Commission Decision 97/747/EC of 27 October 1997 fixing the levels and frequencies of sampling provided for by Council Directive 96/23/EC for the monitoring of certain substances and residues thereof in certain animal products (OJ L 303, 6.11.1997, p. 12).
(2) https://ec.europa.eu/food/sites/food/files/safety/docs/cs_contaminants_catalogue_dioxins_guidance-sampling_exemples-dec2006_en.pdf
(3) The principles as described in the ‘Guidance Document on Measurement Uncertainty for Laboratories performing PCDD/F and PCB Analysis using Isotope Dilution Mass Spectrometry’ [link to website] shall be followed when applicable.
(4) The duplicate analysis is necessary if the result of the first determination is non-compliant. The duplicate analysis is necessary to exclude the possibility of internal cross-contamination or an accidental mix-up of samples. In case the analysis is performed in the course of a contamination incident, confirmation by duplicate analysis might be omitted in case the samples selected for analysis are through traceability linked to the contamination incident and the level found is significantly above the maximum level.
(5) Guidance Document on Measurement Uncertainty for Laboratories performing PCDD/F and PCB Analysis using Isotope Dilution Mass Spectrometry [link to website], Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food [link to website].
(6) The duplicate analysis is necessary if the result of the first determination applying confirmatory methods with the use of 13C-labelled internal standard for the relevant analytes is non-compliant. The duplicate analysis is necessary to exclude the possibility of internal cross-contamination or an accidental mix-up of samples. In case the analysis is performed in the course of a contamination incident, confirmation by duplicate analysis might be omitted in case the samples selected for analysis are through traceability linked to the contamination incident and the level found is significantly above the maximum level.
(7) Identical explanation and requirements for duplicate analysis for control of action levels as in footnote 6 for maximum levels.
ANNEX III
SAMPLE PREPARATION AND REQUIREMENTS FOR METHODS OF ANALYSIS USED IN CONTROL OF THE LEVELS OF DIOXINS (PCDD/FS) AND DIOXIN-LIKE PCBs IN CERTAIN FOODSTUFFS
1. FIELD OF APPLICATION
The requirements set out in this Annex shall be applied where foodstuffs are analysed for the official control of the levels of 2,3,7,8-substituted polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (dioxin-like PCBs) and as regards sample preparation and analytical requirements for other regulatory purposes, including the controls performed by the food business operator to ensure compliance with provisions in Article 4 of Regulation (EC) No 852/2004.
Monitoring for the presence of PCDD/Fs and dioxin-like PCBs in foodstuffs may be performed with two different types of analytical methods:
(a) Screening methods
The goal of screening methods is to select those samples with levels of PCDD/Fs and dioxin-like PCBs that exceed the maximum levels or the action levels. Screening methods shall ensure cost-effective high sample-throughput, thus increasing the chance to discover new incidents where high exposure may lead to health risks for consumers. Their application shall aim to avoid false-compliant results. They may comprise bioanalytical and GC/MS methods.
Screening methods compare the analytical result with a cut-off value, providing a yes/no-decision over the possible exceedance of the maximum or action level. The concentration of PCDD/Fs and the sum of PCDD/Fs and dioxin-like PCBs in samples suspected to be non-compliant with the maximum level must be determined or confirmed by a confirmatory method.
In addition, screening methods may give an indication of the levels of PCDD/Fs and dioxin-like-PCBs present in the sample. In case of application of bioanalytical screening methods the result is expressed as Bioanalytical Equivalents (BEQ), whereas in case of application of physico-chemical GC-MS methods it is expressed as Toxic Equivalents (TEQ). The numerically indicated results of screening methods are suitable for demonstrating compliance or suspected non-compliance or exceedance of action levels and give an indication of the range of levels in case of follow-up by confirmatory methods. They are not suitable for purposes such as evaluation of background levels, estimation of intake, following of time trends in levels or re-evaluation of action and maximum levels.
(b) Confirmatory methods
Confirmatory methods allow the unequivocal identification and quantification of PCDD/Fs and dioxin-like PCBs present in a sample and provide full information on congener basis. Therefore, those methods allow the control of maximum and action levels, including the confirmation of results obtained by screening methods. Furthermore, results may be used for other purposes such as determination of low background levels in food monitoring, following of time trends, exposure assessment of the population and building of a database for possible re-evaluation of action and maximum levels. They are also important for establishing congener patterns in order to identify the source of a possible contamination. Such methods utilise GC-HRMS. For confirming compliance or non-compliance with the maximum level, also GC-MS/MS can be used.
2. BACKGROUND
For calculation of TEQ concentrations, the concentrations of the individual substances in a given sample shall be multiplied by their respective TEF, as established by the World Health Organisation and listed in the Appendix to this Annex, and subsequently summed to give the total concentration of dioxin-like compounds expressed as TEQs.
Screening and confirmatory methods may only be applied for control of a certain matrix if the methods are sensitive enough to detect levels reliably at the maximum or action level.
3. QUALITY ASSURANCE REQUIREMENTS
|
— |
Measures must be taken to avoid cross-contamination at each stage of the sampling and analysis procedure. |
|
— |
The samples must be stored and transported in glass, aluminum, polypropylene or polyethylene containers suitable for storage without any influence on the levels of PCDD/Fs and dioxin-like PCBs in the samples. Traces of paper dust must be removed from the sample container. |
|
— |
The sample storage and transportation has to be performed in a way that maintains the integrity of the foodstuff sample. |
|
— |
Insofar as relevant, finely grind and mix thoroughly each laboratory sample using a process that has been demonstrated to achieve complete homogenisation (e.g. ground to pass a 1 mm sieve); samples have to be dried before grinding if moisture content is too high. |
|
— |
Control of reagents, glassware and equipment for possible influence of TEQ- or BEQ-based results is of general importance. |
|
— |
A blank analysis shall be performed by carrying out the entire analytical procedure omitting only the sample. |
|
— |
For bioanalytical methods, it is of great importance that all glassware and solvents used in analysis shall be tested to be free of compounds that interfere with the detection of target compounds in the working range. Glassware shall be rinsed with solvents or/and heated at temperatures suitable to remove traces of PCDD/Fs, dioxin-like compounds and interfering compounds from its surface. |
|
— |
Sample quantity used for the extraction must be sufficient to fulfill the requirements with respect to a sufficiently low working range including the concentrations of maximum or action levels. |
|
— |
The specific sample preparation procedures used for the products under consideration shall follow internationally accepted guidelines. |
|
— |
In the case of fish, the skin has to be removed as the maximum level applies to muscle meat without skin. However it is necessary that all remaining muscle meat and fat tissue on the inner side of the skin are carefully and completely scraped off from the skin and added to the sample to be analysed. |
4. REQUIREMENTS FOR LABORATORIES
|
— |
In accordance with the provisions of Regulation (EC) No 882/2004, laboratories shall be accredited by a recognised body operating in accordance with ISO Guide 58 to ensure that they are applying analytical quality assurance. Laboratories shall be accredited following the EN ISO/IEC 17025 standard. The principles as described in the Technical Guidelines for the estimation of measurement uncertainty and limits of quantification for PCDD/F and PCB analysis shall be followed when applicable (1). |
|
— |
Laboratory proficiency shall be proven by the continuous successful participation in interlaboratory studies for the determination of PCDD/Fs and dioxin-like PCBs in relevant food matrices and concentration ranges. |
|
— |
Laboratories applying screening methods for routine control of samples shall establish a close cooperation with laboratories applying the confirmatory method, both for quality control and confirmation of the analytical result of suspected samples. |
5. BASIC REQUIREMENTS TO BE MET BY ANALYTICAL PROCEDURE FOR DIOXINS (PCDD/FS) AND DIOXIN-LIKE PCBS
5.1. Low working range and limits of quantification
|
— |
For PCDD/Fs, detectable quantities have to be in the upper femtogram (10– 15 g) range because of extreme toxicity of some of these compounds. For most PCB congeners limit of quantification in the nanogram (10– 9 g) range is already sufficient. However, for the measurement of the more toxic dioxin-like PCB congeners (in particular non-ortho-substituted congeners) the lower end of the working range must reach the low picogram (10– 12 g) levels. |
5.2. High selectivity (specificity)
|
— |
A distinction is required between PCDD/Fs and dioxin-like PCBs and a multitude of other, coextracted and possibly interfering compounds present at concentrations up to several orders of magnitude higher than those of the analytes of interest. For gas chromatography/mass spectrometry (GC-MS) methods, a differentiation among various congeners is necessary, such as between toxic (e.g. the seventeen 2,3,7,8-substituted PCDD/Fs, and twelve dioxin-like PCBs) and other congeners. |
|
— |
Bioanalytical methods shall be able to detect the target compounds as the sum of PCDD/Fs, and/or dioxin-like PCBs. Sample clean-up shall aim at removing compounds causing false non-compliant results or compounds that may decrease the response, causing false-compliant results. |
5.3. High accuracy (trueness and precision, bioassay apparent recovery)
|
— |
For GC-MS methods, the determination shall provide a valid estimate of the true concentration in a sample. High accuracy (accuracy of the measurement: the closeness of the agreement between the result of a measurement with the true or assigned value of the measurand) is necessary to avoid the rejection of a sample analysis result on the basis of poor reliability of the determined TEQ level. Accuracy is expressed as trueness (difference between the mean value measured for an analyte in a certified material and its certified value, expressed as percentage of this value) and precision (RSDR relative standard deviation calculated from results generated under reproducibility conditions). |
|
— |
For bioanalytical methods, the bioassay apparent recovery shall be determined. |
5.4. Validation in the range of maximum level and general quality control measures
|
— |
Laboratories shall demonstrate the performance of a method in the range of the maximum level, e.g. 0,5×, 1× and 2× the maximum level with an acceptable coefficient of variation for repeated analysis, during the validation procedure and/or during routine analysis. |
|
— |
Regular blank controls and spiking experiments or analysis of control samples (preferably, if available, certified reference material) shall be performed as internal quality control measures. Quality control (QC) charts for blank controls, spiking experiments or analysis of control samples shall be recorded and checked to make sure the analytical performance is in accordance with the requirements. |
5.5. Limit of quantification
|
— |
For a bioanalytical screening method, establishment of the LOQ is not an indispensable requirement but the method shall prove that it can differentiate between the blank and the cut-off value. When providing a BEQ-level, a reporting level shall be established to deal with samples showing a response below this level. The reporting level shall be demonstrated to be different from procedure blank samples at least by a factor of three, with a response below the working range. It shall therefore be calculated from samples containing the target compounds around the required minimum level, and not from a S/N ratio or an assay blank. |
|
— |
Limit of quantification (LOQ) for a confirmatory method shall be about one fifth of the maximum level. |
5.6. Analytical criteria
|
— |
For reliable results from confirmatory or screening methods, the following criteria must be met in the range of the maximum level for the TEQ value respectively the BEQ value, whether determined as total TEQ or total BEQ (as sum of PCDD/F and dioxin-like PCBs) or separately for PCDD/Fs and dioxin-like PCBs. |
|
|
Screening with bioanalytical or physico-chemical methods |
Confirmatory methods |
|
False-compliant rate (*1) |
< 5 % |
|
|
Trueness |
|
– 20 % to + 20 % |
|
Repeatability (RSDr) |
< 20 % |
|
|
Intermediate precision (RSDR) |
< 25 % |
< 15 % |
5.7. Specific requirements for screening methods
|
— |
Both GC-MS and bioanalytical methods may be used for screening. For GC-MS methods the requirements as laid down in point 6 are to be used. For cell-based bioanalytical methods specific requirements are laid down in point 7. |
|
— |
Laboratories applying screening methods for routine control of samples shall establish a close cooperation with laboratories applying the confirmatory method. |
|
— |
Performance verification of the screening method is required during routine analysis, by analytical quality control and ongoing method validation. There must be a continuous programme for control of compliant results. |
|
— |
Check on possible suppression of the cell response and cytotoxicity. 20 % of the sample extracts shall be measured in routine screening without and with TCDD added corresponding to the maximum or action level, to check if the response is possibly suppressed by interfering substances present in the sample extract. The measured concentration of the spiked sample is compared to the sum of the concentration of the unspiked extract plus the spiking concentration. If this measured concentration is more than 25 % lower than the calculated (sum) concentration, this is an indication of a potential signal suppression and the respective sample must be submitted to confirmatory analysis. Results shall be monitored in quality control charts. |
|
— |
Quality control on compliant samples Approximately 2 % to 10 % of the compliant samples, depending on sample matrix and laboratory experience, shall be confirmed. |
|
— |
Determination of false-compliant rates from QC data The rate of false-compliant results from screening of samples below and above the maximum level or the action level shall be determined. Actual false-compliant rates shall be below 5 %. After a minimum of 20 confirmed results per matrix/matrix group is available from the quality control of compliant samples, conclusions on the false-compliant rate shall be drawn from this database. The results from samples analysed in ring trials or during contamination incidents, covering a concentration range up to, e.g. 2× the maximum level (ML), may also be included in the minimum of 20 results for evaluation of the false-compliant rate. The samples shall cover most frequent congener patterns, representing various sources. Although screening assays shall preferentially aim to detect samples exceeding the action level, the criterion for determining false-compliant rates is the maximum level, taking into account the expanded measurement uncertainty of the confirmatory method. |
|
— |
Potential non-compliant results from screening shall always be verified by a full re-analysis of the original sample by a confirmatory method. These samples may also be used to evaluate the rate of false non-compliant results. For screening methods, the rate of false non-compliant results is the fraction of results confirmed to be compliant from confirmatory analysis, while in previous screening the sample had been declared to be suspected to be non-compliant. However, evaluation of the advantageousness of the screening method shall be based on comparison of false non-compliant samples with the total number of samples checked. This rate shall be low enough to make the use of a screening tool advantageous. |
|
— |
At least under validation conditions, bioanalytical methods shall provide a valid indication of the TEQ level, calculated and expressed as BEQ. |
|
— |
Also for bioanalytical methods carried out under repeatability conditions, the intra-laboratory RSDr would typically be smaller than the reproducibility RSDR. |
6. SPECIFIC REQUIREMENTS FOR GC-MS METHODS TO BE COMPLIED WITH FOR SCREENING OR CONFIRMATORY PURPOSES
6.1. Acceptable differences between upperbound and lowerbound WHO-TEQ levels
|
— |
The difference between upperbound level and lowerbound level shall not exceed 20 % for confirmation of the exceedance of maximum or in case of need of action levels. |
6.2. Control of recoveries
|
— |
Addition of 13C-labelled 2,3,7,8-chlorine-substituted internal PCDD/F standards and of 13C-labelled internal dioxin-like PCB standards must be carried out at the very beginning of the analytical method, e.g. prior to extraction, in order to validate the analytical procedure. At least one congener for each of the tetra- to octa-chlorinated homologous groups for PCDD/Fs and at least one congener for each of the homologous groups for dioxin-like PCBs must be added (alternatively, at least one congener for each mass spectrometric selected ion recording function used for monitoring PCDD/Fs and dioxin-like PCBs). In case of confirmatory methods, all seventeen 13C-labelled 2,3,7,8-substituted internal PCDD/F standards and all twelve 13C-labelled internal dioxin-like PCB standards shall be used. |
|
— |
Relative response factors shall also be determined for those congeners for which no 13C-labelled analogue is added by using appropriate calibration solutions. |
|
— |
For foodstuffs of plant origin and foodstuffs of animal origin containing less than 10 % fat, the addition of the internal standards is mandatory prior to extraction. For foodstuffs of animal origin containing more than 10 % fat, the internal standards may be added either before or after fat extraction. An appropriate validation of the extraction efficiency shall be carried out, depending on the stage at which internal standards are introduced and on whether results are reported on product or fat basis. |
|
— |
Prior to GC-MS analysis, one or two recovery (surrogate) standard(s) must be added. |
|
— |
Control of recovery is necessary. For confirmatory methods, the recoveries of the individual internal standards shall be in the range of 60 to 120 %. Lower or higher recoveries for individual congeners, in particular for some hepta- and octa- chlorinated dibenzo-p-dioxins and dibenzofurans, are acceptable on the condition that their contribution to the TEQ value does not exceed 10 % of the total TEQ value (based on sum of PCDD/F and dioxin-like PCBs). For GC-MS screening methods, the recoveries shall be in the range of 30 to 140 %. |
6.3. Removal of interfering substances
|
— |
Separation of PCDD/Fs from interfering chlorinated compounds such as non-dioxin-like PCBs and chlorinated diphenyl ethers shall be carried out by suitable chromatographic techniques (preferably with a florisil, alumina and/or carbon column). |
|
— |
Gas-chromatographic separation of isomers shall be sufficient (< 25 % peak to peak between 1,2,3,4,7,8-HxCDF and 1,2,3,6,7,8-HxCDF). |
6.4. Calibration with standard curve
|
— |
The range of the calibration curve shall cover the relevant range of maximum or action levels. |
6.5. Specific criteria for confirmatory methods
|
— |
For GC-HRMS:
|
|
— |
For GC-MS/MS:
|
7. SPECIFIC REQUIREMENTS FOR BIOANALYTICAL METHODS
Bioanalytical methods are methods based on the use of biological principles like cell-based assays, receptor-assays or immunoassays. This point establishes requirements for bioanalytical methods in general.
A screening method in principle classifies a sample as compliant or suspected to be non-compliant. For this, the calculated BEQ level is compared to the cut-off value (see point 7.3). Samples below the cut-off value are declared compliant, samples equal or above the cut-off value as suspected to be non-compliant, requiring analysis by a confirmatory method. In practice, a BEQ level corresponding to two-thirds of the maximum level may serve as cut-off value provided that a false-compliant rate below 5 % and an acceptable rate for false non-compliant results are ensured. With separate maximum levels for PCDD/Fs and for the sum of PCDD/Fs and dioxin-like PCBs, checking compliance of samples without fractionation requires appropriate bioassay cut-off values for PCDD/Fs. For checking of samples exceeding the action levels, an appropriate percentage of the respective action level would suit as cut-off value.
If an indicative level is expressed in BEQs, the results from the the sample must be given in the working range and exceeding the reporting limit (see points 7.1.1 and 7.1.6).
7.1. Evaluation of the test response
7.1.1. General requirements
|
— |
When calculating the concentrations from a TCDD calibration curve, values at the higher end of the curve will show a high variation (high coefficient of variation (CV)). The working range is the area where this CV is smaller than 15 %. The lower end of the working range (reporting limit) must further be set significantly (at least by a factor of three) above the procedure blanks. The upper end of the working range is usually represented by the EC70 value (70 % of maximal effective concentration), but lower if the CV is higher than 15 % in this range. The working range shall be established during validation. Cut-off values (see point 7.3) must be within the working range. |
|
— |
Standard solutions and sample extracts shall be tested in triplicate or at least in duplicate. When using duplicates, a standard solution or a control extract tested in four to six wells divided over the plate shall produce a response or concentration (only possible in the working range) based on a CV < 15 %. |
7.1.2. Calibration
7.1.2.1. Calibration with standard curve
|
— |
Levels in samples may be estimated by comparison of the test response with a calibration curve of TCDD (or PCB 126 or a PCDD/F/dioxin-like PCB standard mixture) to calculate the BEQ level in the extract and subsequently in the sample. |
|
— |
Calibration curves shall contain 8 to 12 concentrations (at least in duplicates), with enough concentrations in the lower part of the curve (working range). Special attention shall be paid to the quality of the curve-fit in the working range. As such, the R2 value is of little or no value in estimating the goodness of fit in nonlinear regression. A better fit will be achieved by minimising the difference between calculated and observed levels in the working range of the curve (e.g. by minimising the sum of squared residuals). |
|
— |
The estimated level in the sample extract is subsequently corrected for the BEQ level calculated for a matrix or solvent blank sample (to account for impurities from solvents and chemicals used), and the apparent recovery (calculated from the BEQ level of suitable reference samples with representative congener patterns around the maximum or action level). For performing a recovery correction, the apparent recovery must always be within the required range (see point 7.1.4). Reference samples used for recovery correction must comply with requirements as given in point 7.2. |
7.1.2.2. Calibration with reference samples
Alternatively, a calibration curve prepared from at least four reference samples (see point 7.2: one matrix blank, plus three reference samples at 0,5×, 1,0× and 2,0× the maximum or action level may be used, eliminating the need to correct for blank and recovery if matrix properties of the reference samples match those of the unknown samples. In this case, the test response corresponding to two-thirds of the maximum level (see point 7.3) may be calculated directly from these samples and used as cut-off value. For checking of samples exceeding the action levels, an appropriate percentage of these action levels would suit as cut-off value.
7.1.3. Separate determination of PCDD/Fs and dioxin-like PCBs
Extracts may be split into fractions containing PCDD/Fs and dioxin-like PCBs, allowing a separate indication of PCDD/Fs and dioxin-like PCB TEQ levels (in BEQs). A PCB 126 standard calibration curve shall preferentially be used to evaluate results for the fraction containing dioxin-like PCBs.
7.1.4. Bioassay apparent recoveries
The ‘bioassay apparent recovery’ shall be calculated from suitable reference samples with representative congener patterns around the maximum or action level and expressed as percentage of the BEQ level in comparison to the TEQ level. Depending on the type of assay and TEFs (2) used, the differences between TEF and REP factors for dioxin-like PCBs may cause low apparent recoveries for dioxin-like PCBs in comparison to PCDD/Fs. Therefore, if a separate determination of PCDD/Fs and dioxin-like PCBs is performed, bioassay apparent recoveries shall be: for dioxin-like PCBs 20 % to 60 %, for PCDD/Fs 50 % to 130 % (ranges apply for TCDD calibration curve). As the contribution of dioxin-like PCBs to the sum of PCDD/Fs and dioxin-like PCBs may vary between different matrices and samples, bioassay apparent recoveries for the sum parameter reflect these ranges and shall be between 30 % to 130 %.
7.1.5. Control of recoveries for clean-up
The loss of compounds during the clean-up shall be checked during validation. A blank sample spiked with a mixture of the different congeners shall be submitted to clean-up (at least n = 3) and the recovery and variability checked by a confirmatory method. The recovery shall be within 60 to 120 % especially for congeners contributing more than 10 % to the TEQ-level in various mixtures.
7.1.6. Reporting Limit
When reporting BEQ levels, a reporting limit shall be determined from relevant matrix samples involving typical congener patterns, but not from the calibration curve of the standards due to low precision in the lower range of the curve. Effects from extraction and clean-up must be taken into account. The reporting limit must be set significantly (at least by a factor of three) above the procedure blanks.
7.2. Use of reference samples
|
— |
Reference samples shall represent sample matrix, congener patterns and concentration ranges for PCDD/Fs and dioxin-like PCBs around the maximum or action level. |
|
— |
A procedure blank, or preferably a matrix blank, and a reference sample at the maximum or action level have to be included in each test series. These samples must be extracted and tested at the same time under identical conditions. The reference sample must show a clearly elevated response in comparison to the blank sample, thus ensuring the suitability of the test. Those samples may be used for blank and recovery corrections. |
|
— |
Reference samples chosen for performing a recovery correction shall be representative for the test samples, meaning that congener patterns shall not lead to an underestimation of levels. |
|
— |
Extra reference samples at, e.g. 0,5× and 2× the maximum or action level may be included to demonstrate the proper performance of the test in the range of interest for the control of the maximum or action level. Combined, these samples may be used for calculating the BEQ-levels in test samples (see point 7.1.2.2). |
7.3. Determination of cut-off values
The relationship between bioanalytical results in BEQ and results from confirmatory methods in TEQ shall be established (e.g. by matrix-matched calibration experiments, involving reference samples spiked at 0, 0,5×, 1× and 2× the maximum level (ML), with six repetitions on each level (n = 24)). Correction factors (blank and recovery) may be estimated from this relationship but shall be checked in each test series by including procedure/matrix blanks and recovery samples (see point 7.2).
Cut-off values shall be established for decision over sample compliance with maximum levels or for control of action levels, if of interest, with the respective maximum or action levels set for either PCDD/Fs and dioxin-like PCBs alone, or for the sum of PCDD/Fs and dioxin-like PCBs. They are represented by thelower endpoint of the distribution of bioanalytical results (corrected for blank and recovery) corresponding to the decision limit of the confirmatory method based on a 95 % level of confidence, implying a false-compliant rate < 5 %, and on a RSDR < 25 %. The decision limit of the confirmatory method is the maximum level, taking into account the expanded measurement uncertainty.
In practice, the cut-off value (in BEQ) may be calculated from the following approaches (see Figure 1):
7.3.1. Use of the lower band of the 95 % prediction interval at the decision limit of the confirmatory method
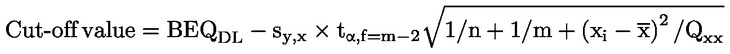
with:
|
BEQDL |
BEQ corresponding to the decision limit of the confirmatory method, being the ML taking into account the expanded measurement uncertainty |
|
sy,x |
residual standard deviation |
|
t α,f = m – 2 |
student factor (α = 5 %, f = degrees of freedom, single-sided) |
|
m |
total number of calibration points (index j) |
|
n |
number of repetitions on each level |
|
xi |
sample concentration (in TEQ) of calibration point I determined by a confirmatory method |
|
|
mean of the concentrations (in TEQ) of all calibration samples |
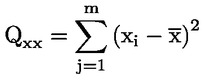 square sum parameter
square sum parameter
|
i |
= |
index for calibration point i |
7.3.2. Calculation from bioanalytical results (corrected for blank and recovery) of multiple analyses of samples (n ≥ 6) contaminated at the decision limit of the confirmatory method, as the lower endpoint of the data distribution at the corresponding mean BEQ value:
Cut-off value = BEQDL – 1,64 × SDR
with
|
SDR |
standard deviation of bioassay results at BEQDL, measured under within-laboratory reproducibility conditions |
7.3.3. Calculation as mean value of bioanalytical results (in BEQ, corrected for blank and recovery) from multiple analysis of samples (n ≥ 6) contaminated at two-thirds of the maximum or action level. This is based on the observation that this level will be around the cut-off determined under point 7.3.1 or 7.3.2.
Calculation of cut-off values based on a 95 % level of confidence implying a false-compliant rate < 5 %, and a RSDR < 25 %:
|
1. |
from the lower band of the 95 % prediction interval at the decision limit of the confirmatory method, |
|
2. |
from multiple analysis of samples (n ≥ 6) contaminated at the decision limit of the confirmatory method as the lower endpoint of the data distribution (represented in the figure by a bell-shaped curve) at the corresponding mean BEQ value. |
Figure 1
7.3.4. Restrictions to cut-off values
BEQ-based cut-off values calculated from the RSDR achieved during validation using a limited number of samples with different matrix/congener patterns may be higher than the TEQ-based maximum or action levels due to a better precision than attainable in routine when an unknown spectrum of possible congener patterns has to be controlled. In such cases, cut-off values shall be calculated from an RSDR = 25 %, or two-thirds of the maximum or action level shall be preferred.
7.4. Performance characteristics
|
— |
Since no internal standards can be used in bioanalytical methods, tests on repeatability shall be carried out to obtain information on the standard deviation within and between test series. Repeatability shall be below 20 % and intra-laboratory reproducibility shall be below 25 %. This shall be based on the calculated levels in BEQs after blank and recovery correction. |
|
— |
As part of the validation process, the test must be shown to discriminate between a blank sample and a level at the cut-off value, allowing the identification of samples above the corresponding cut-off value (see point 7.1.2). |
|
— |
Target compounds, possible interferences and maximum tolerable blank levels shall be defined. |
|
— |
The per cent standard deviation in the response or concentration calculated from the response (only possible in working range) of a triplicate determination of a sample extract shall not be above 15 %. |
|
— |
The uncorrected results of the reference sample(s) expressed in BEQs (blank and at the maximum or action level) shall be used for evaluation of the performance of the bioanalytical method over a constant time period. |
|
— |
QCcharts for procedure blanks and each type of reference sample shall be recorded and checked to make sure the analytical performance is in accordance with the requirements, in particular for the procedure blanks with regard to the requested minimum difference to the lower end of the working range and for the reference samples with regard to within-laboratory reproducibility. Procedure blanks must be well controlled in order to avoid false-compliant results when subtracted. |
|
— |
The results from the confirmatory methods of suspected samples and 2 to 10 % of the compliant samples (minimum of 20 samples per matrix) shall be collected and used to evaluate the performance of the screening method and the relationship between BEQs and TEQs. This database might be used for re-evaluation of cut-off values applicable to routine samples for the validated matrices. |
|
— |
Successful method performance may also be demonstrated by participation in ring trials. The results from samples analysed in ring trials, covering a concentration range up to, e.g. 2× ML, may also be included in the evaluation of the false-compliant rate, if a laboratory is able to demonstrate its successful performance. The samples shall cover most frequent congener patterns, representing various sources. |
|
— |
During incidents, the cut-off values may be re-evaluated, reflecting the specific matrix and congener patterns of this single incident. |
8. REPORTING OF THE RESULT
Confirmatory methods
|
— |
The analytical results shall contain the levels of the individual PCDD/F and dioxin-like PCB congeners and TEQ-values shall be reported as lower-bound, upper-bound and medium-bound in order to include a maximum of information in the reporting of the results and thereby enabling the interpretation of the results according to specific requirements. |
|
— |
The report shall also include the method used for extraction of PCDD/Fs, dioxin-like PCBs and lipids. The lipid content of the sample shall be determined and reported for food matrices with maximum levels expressed on fat basis and with an expected fat concentration in the range of 0-2 % (in correspondence to existing legislation). For other samples, the determination of the lipid content is optional. |
|
— |
The recoveries of the individual internal standards must be made available in case the recoveries are outside the range mentioned in point 6.2 where the maximum level is exceeded (in this case, the recoveries for one of the two duplicate analysis) and in other cases upon request. |
|
— |
As the expanded measurement uncertainty is to be taken into account when deciding about the compliance of a sample, this parameter shall also be made available. Thus, analytical results shall be reported as x +/– U whereby x is the analytical result and U is the expanded measurement uncertainty using a coverage factor of 2 which gives a level of confidence of approximately 95 %. In case of a separate determination of PCDD/Fs and dioxin-like-PCBs the sum of the estimated expanded uncertainty of the separate analytical results of PCDD/Fs and dioxin-like PCBs has to be used for the sum of PCDD/Fs and dioxin-like PCBs. |
|
— |
The results shall be expressed in the same units and with the same number of significant figures as the maximum levels laid down in Regulation (EC) No 1881/2006. |
Bioanalytical screening methods
|
— |
The result of the screening shall be expressed as compliant or suspected to be non-compliant (‘suspected’). |
|
— |
In addition, an indicative result for PCDD/F and/or dioxin-like PCBs expressed in BEQ (not TEQ) may be given (see point 1). Samples with a response below the reporting limit shall be expressed as lower than the reporting limit. Samples with a response above the working range shall be reported as exceeding the working range and the level corresponding to the upper end of the working range shall be given in BEQ. |
|
— |
For each type of sample matrix, the report shall mention the maximum or action level on which the evaluation is based. |
|
— |
The report shall mention the type of test applied, the basic test principle and kind of calibration. |
|
— |
The report shall also include the method used for extraction of PCDD/Fs, dioxin-like PCBs and lipids. The lipid content of the sample shall be determined and reported for food matrices with maximum levels expressed on fat basis and with an expected fat concentration in the range of 0-2 % (in correspondence to existing legislation). For other samples, the determination of the lipid content is optional. |
|
— |
In the case of samples suspected to be non-compliant, the report needs to include a note on the action to be taken. The concentration of PCDD/Fs and the sum of PCDD/Fs and dioxin-like PCBs in those samples with elevated levels has to be determined/confirmed by a confirmatory method. |
|
— |
Non-compliant results shall only be reported from confirmatory analysis. |
Physico-chemical screening methods
|
— |
The result of the screening shall be expressed as compliant or suspected to be non-compliant (‘suspected’). |
|
— |
For each type of sample matrix, the report shall mention the maximum or action level on which the evaluation is based. |
|
— |
In addition, levels for individual PCDD/F and/or dioxin-like PCB congeners and TEQ-values reported as lower-bound, upper-bound and medium-bound may be given. The results shall be expressed in the same units and with (at least) the same number of significant figures as the maximum levels laid down in Regulation (EC) No 1881/2006. |
|
— |
The recoveries of the individual internal standards must be made available in case the recoveries are outside the range mentioned in point 6.2 and in other cases upon request. |
|
— |
The report shall mention the GC-MS method applied. |
|
— |
The report shall also include the method used for extraction of PCDD/Fs, dioxin-like PCBs and lipids. The lipid content of the sample shall be determined and reported for food matrices with maximum levels expressed on fat basis and with an expected fat concentration in the range of 0-2 % (in correspondence to existing legislation). For other samples, the determination of the lipid content is optional. |
|
— |
In case of samples suspected to be non-compliant, the report needs to include a note on the action to be taken. The concentration of PCDD/Fs and the sum of PCDD/Fs and dioxin-like PCBs in those samples with elevated levels has to be determined/confirmed by a confirmatory method. |
|
— |
Non-compliance can only be decided after confirmatory analysis. |
(1) Guidance Document on Measurement Uncertainty for Laboratories performing PCDD/F and PCB Analysis using Isotope Dilution Mass Spectrometry [link to website], Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food [link to website].
(*1) With respect to the maximum levels
(2) Current requirements are based on the TEFs published in: M. Van den Berg et al, Toxicol Sci 93 (2), 223-241 (2006).
Appendix
WHO-TEFs for human risk assessment based on the conclusions of the World Health Organisation (WHO) 0151 International Programme on Chemical Safety (IPCS) expert meeting which was held in Geneva in June 2005 (1)
|
Congener |
TEF value |
Congener |
TEF value |
||
|
Dibenzo-p-dioxins (‘PCDDs’) |
‘Dioxin-like’ PCBs Non-ortho PCBs + Mono-ortho PCBs |
||||
|
2,3,7,8-TCDD |
1 |
|
|
||
|
1,2,3,7,8-PeCDD |
1 |
Non-ortho PCBs |
|
||
|
1,2,3,4,7,8-HxCDD |
0,1 |
PCB 77 |
0,0001 |
||
|
1,2,3,6,7,8-HxCDD |
0,1 |
PCB 81 |
0,0003 |
||
|
1,2,3,7,8,9-HxCDD |
0,1 |
PCB 126 |
0,1 |
||
|
1,2,3,4,6,7,8-HpCDD |
0,01 |
PCB 169 |
0,03 |
||
|
OCDD |
0,0003 |
|
|
||
|
Dibenzofurans (‘PCDFs’) |
Mono-ortho PCBs |
||||
|
2,3,7,8-TCDF |
0,1 |
PCB 105 |
0,00003 |
||
|
1,2,3,7,8-PeCDF |
0,03 |
PCB 114 |
0,00003 |
||
|
2,3,4,7,8-PeCDF |
0,3 |
PCB 118 |
0,00003 |
||
|
1,2,3,4,7,8-HxCDF |
0,1 |
PCB 123 |
0,00003 |
||
|
1,2,3,6,7,8-HxCDF |
0,1 |
PCB 156 |
0,00003 |
||
|
1,2,3,7,8,9-HxCDF |
0,1 |
PCB 157 |
0,00003 |
||
|
2,3,4,6,7,8-HxCDF |
0,1 |
PCB 167 |
0,00003 |
||
|
1,2,3,4,6,7,8-HpCDF |
0,01 |
PCB 189 |
0,00003 |
||
|
1,2,3,4,7,8,9-HpCDF |
0,01 |
|
|
||
|
OCDF |
0,0003 |
|
|
||
|
|
|||||
(1) Martin van den Berg et al., The 2005 World Health Organisation Re-evaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-like Compounds. Toxicological Sciences 93(2), 223–241 (2006).
ANNEX IV
SAMPLE PREPARATION AND REQUIREMENTS FOR METHODS OF ANALYSIS USED IN CONTROL OF THE LEVELS OF NON-DIOXIN-LIKE PCBS IN CERTAIN FOODSTUFFS
The requirements set out in this Annex shall be applied where foodstuffs are analysed for the official control of the levels of non-dioxin-like PCBs and as regards sample preparation and analytical requirements for other regulatory purposes, including the controls performed by the food business operator to ensure compliance with the provisions in Article 4 of Regulation (EC) No 852/2004.
The provisions on sample preparation provided for in point 3 of Annex III of this Regulation shall also be applicable for the control of the levels of non-dioxin-like PCBs in food.
1. Applicable detection methods
Gas Chromatography/Electron Capture Detection (GC-ECD), GC-LRMS, GC-MS/MS, GC-HRMS or equivalent methods.
2. Identification and confirmation of analytes of interest:
|
— |
Relative retention time in relation to internal standards or reference standards (acceptable deviation of +/– 0,25 %). |
|
— |
Gas chromatographic separation of the non-dioxin-like PCBs (from interfering substances, especially co-eluting PCBs, in particular if levels of samples are in the range of legal limits and non-compliance is to be confirmed (1)). |
|
— |
For GC-MS techniques:
|
|
— |
For GC-ECD: Confirmation of results exceeding the maximum level with two GC columns with stationary phases of different polarity. |
3. Demonstration of performance of method:
Validation in the range of the maximum level (0,5 to 2 times the maximum level) with an acceptable coefficient of variation for repeated analysis (see requirements for intermediate precision in point 8).
4. Limit of quantification:
The sum of the LOQs (2) of non-dioxin-like PCBs shall not be higher than one-third of the maximum level (3).
5. Quality control:
Regular blank controls, analysis of spiked samples, quality control samples, participation in interlaboratory studies on relevant matrices.
6. Control of recoveries:
|
— |
Use of suitable internal standards with physico-chemical properties comparable to analytes of interest. |
|
— |
Addition of internal standards:
|
|
— |
Requirements for methods using all six isotope-labelled non-dioxin-like PCB congeners:
|
|
— |
Requirements for methods using not all six isotope-labelled internal standards or other internal standards:
|
|
— |
The recoveries of unlabelled congeners shall be checked by spiked samples or quality control samples with concentrations in the range of the maximum level. Acceptable recoveries for these congeners are between 60 and 120 %. |
7. Requirements for laboratories
In accordance with the provisions of Regulation (EC) No 882/2004, laboratories shall be accredited by a recognised body operating in accordance with ISO Guide 58 to ensure that they are applying analytical quality assurance. Laboratories shall be accredited following the EN ISO/IEC 17025 standard. In addition, the principles as described in Technical Guidelines for the estimation of measurement uncertainty and limits of quantification for PCB analysis shall be followed when applicable (4).
8. Performance characteristics: Criteria for the sum of non-dioxin-like PCBs at the maximum level
|
|
Isotope dilution mass spectrometry (*1) |
Other techniques |
|
Trueness |
– 20 to + 20 % |
– 30 to + 30 % |
|
Intermediate precision (RSDR) |
≤ 15 % |
≤ 20 % |
|
Difference between upper and lower bound calculation |
≤ 20 % |
≤ 20 % |
9. Reporting of results
|
— |
The analytical results shall contain the levels of the individual non-dioxin-like PCB congeners and the sum of non-dioxin-like PCBs, reported as lower-bound, upper-bound and medium-bound, in order to include a maximum of information in the reporting of the results and thereby enabling the interpretation of the results according to specific requirements. |
|
— |
The report shall also include the method used for the extraction of PCBs and lipids. The lipid content of the sample shall be determined and reported for food matrices with maximum levels expressed on fat basis and with an expected fat concentration in the range of 0-2 % (in correspondence to existing legislation). For other samples, the determination of the lipid content is optional. |
|
— |
The recoveries of the individual internal standards must be made available in case the recoveries are outside the range mentioned in point 6, in case the maximum level is exceeded and in other cases upon request. |
|
— |
As the expanded measurement uncertainty is to be taken into account when deciding about the compliance of a sample, that parameter shall also be made available. Thus, analytical results shall be reported as x +/– U whereby x is the analytical result and U is the expanded measurement uncertainty using a coverage factor of 2 which gives a level of confidence of approximately 95 %. |
|
— |
The results shall be expressed in the same units and with the same number of significant figures as the maximum levels laid down in Regulation (EC) No 1881/2006. |
(1) Congeners often found to co-elute are, e.g. PCB 28/31, PCB 52/69 and PCB 138/163/164. For GC-MS also possible interferences from fragments of higher chlorinated congeners have to be considered.
(2) The principles as described in the ‘Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food’ [link to website] shall be followed when applicable.
(3) It is highly recommendable to have a lower contribution of the reagent blank level to the level of a contaminant in a sample. It is in the responsibility of the laboratory to control the variation of blank levels, in particular, if the blank levels are subtracted.
(4) ‘Guidance Document on Measurement Uncertainty for Laboratories performing PCDD/F and PCB Analysis using Isotope Dilution Mass Spectrometry’ [link to website], ‘Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food’ [link to website].
(*1) Use of all six 13C-labelled analogues as internal standards required